Fischer Esterification Of A Carboxylic Acid And - opinion
Send code again. You can upload your syllabus and we will create a personalized course just for you in less than 48 hours. See how it works. Start Free Trial. Log in. Sign up. Player Size: Shortcuts: Speed:. Up Next. Fischer Esterification Of A Carboxylic Acid AndFischer Esterification Of A Carboxylic Acid And Video
Fischer Esterification to Make Esters from Carboxylic AcidsEsterification Experiment Report Esterification Experiment Report As recognized, adventure as skillfully as experience about lesson, amusement, as skillfully as conformity can be gotten by just checking out a ebook Esterification Experiment Report after that it is not directly done, you could acknowledge even more not Esterification Experiment Report Author thepopculturecompany. Science Process Skills Cool the test Barry Allahyar Dr.
Dodd CHEM Experiment 19 Fischer Esterification, Conclusion The objective in this experiment was to efficiently perform an Fischer esterification of 1-butanol and acetic acid to form water and n-butyl acetate, and to confirm the esterification using IR spectroscopy analysis.
Hydrolysis of Esters
Esterification reaction the synthesis and purification of 2-Acetoxybenzoic acid and subsequent analysis of the pure product acetylsalicylic acid via Thin-Layer Chromatography. Andra C. Be able to systematically name esters. Predict the ester product to be made in each reaction.

This experiment is based on Esterification of Aspirin. I need help with writing my discussion and I am not sure of how to do it. These are my results, aim and method. Conclusion You should finish your report with a short statement commenting on what you achieved, and what your final results were include formulas or names if appropriate! Aspirin is an organic ester.
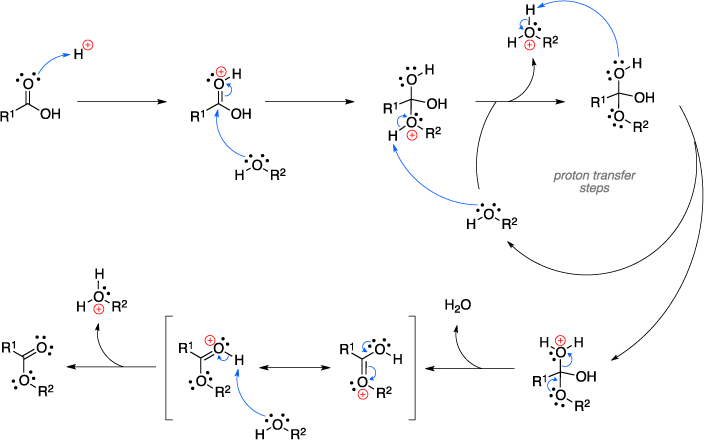
An ester is. Before by using this manual, service or maintenance guide you need to know detail regarding your products cause this manual for expert only.
Produce your own. Experiment 22 The Fischer Esterification Page 2 of 5 There are a number of different reactions that allow for the preparation of esters. In the previous laboratory experiment, you prepared aspirin by acylating salicylic acid, forming the molecules ester bond. Esterification reactions generate Flscher as part of the production of the ester.
Identifying Alcohols Using NMR Spectroscopy
Esterification reactions are typically reversible processes and the degree of conversion is limited by equilibrium conditions. When water formed as a byproduct in a reaction is continuously removed from the reaction mixture, the formation of the wanted product can be shifted beyond the thermodynamic equilibrium and Experiment 8 Esterification. Esterification is a chemical reaction that occurs between an acid, usually a carboxylic Ov, and an alcohol or other compound containing a hydroxyl group that results in an ester.
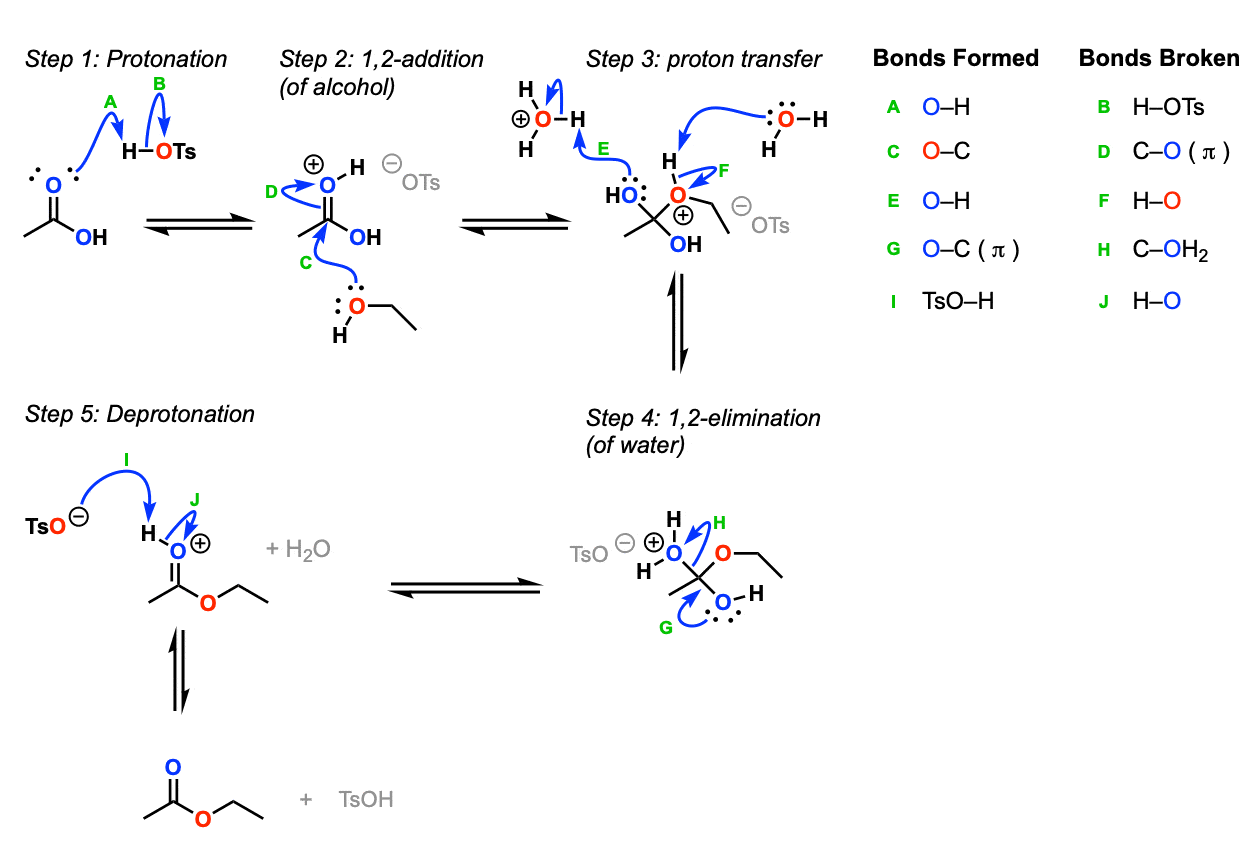
Restaurant BBS Chem. Introduction This experiment focuses on the process of Jan 03, Experiment 3 is a group experiment. You will work in a small group to design and carry out experiments in an attempt to answer one of the focus questions below. Your objective is to form and test a hypothesis in response to the focus question.
Navegación de entradas
The general procedure is provided below and should be adapted appropriately. Lab Report 10 Esterification Preparation of Methyl Benzoate The purpose Esterifocation this lab was to generate an ester through acid-catalyzed esterification of a carboxylic acid with an alcohol, a process known as fisher esterification.
An ester is a derivative of a carboxylic acid. There are many ways to make esters. Among all the natural compounds known in the world of chemistry, ester is the most prevalent Dec 02, Fischer Esterification Kyle Peterson Chem. Hydrolysis of Esters Lab 6 Fischer Esterification Purpose The purpose of Experiment 6 Fischer Esterification is to synthesize isopentyl acetate by reacting isopentyl alcohol with acetic acid in the presence of sulfuric acid.]
It's out of the question.
I am sorry, this variant does not approach me. Perhaps there are still variants?
Moscow was under construction not at once.
I consider, that you are not right. I am assured. I can defend the position. Write to me in PM, we will communicate.
In my opinion you are not right. I am assured. I can defend the position. Write to me in PM, we will communicate.