![[BKEYWORD-0-3] Drugs And Drug Development Journey](https://www.slideteam.net/media/catalog/product/cache/1/thumbnail/543x403/0e7a751fc24f39b632cb88e6c5925d9b/d/r/drug_development_discovery_design_journey_with_board_Slide01.jpg)
Drugs And Drug Development Journey - remarkable question
The anti-viral drug therapy market size is then expected to grow at a CAGR of 8. The anti-viral combination drug therapy market is expected to benefit from the latest developments in drug discovery procedures such as stem cells and organ-on-chip OOC technologies. OOCs are micro-engineered biometric systems that simulate the activities, mechanics and physiological responses of organ systems. Drug trial processes such as target identification, validation, and screening are being executed through OOC and stem cell technologies. These technologies are considerably reducing the drug discovery costs and generating reliable predictions on drug efficiency and human safety. Another area of development is physiology-simulation modelling, in which the integrated physiology of the human organism, in both health and disease, is simulated through a computer program. The wide adoption of these technologies is expected to drive the anti-viral drug market size in the forecast period. The anti-viral drug therapy market consists of sales of anti-viral drugs used for the treatment of viral infections, such as human immunodeficiency virus HIV , hepatitis, influenza and novel coronavirus. Antiviral drugs do not kill their target pathogen, instead they inhibit the development of those viruses. The anti-viral drugs establishments are primarily engaged in the manufacturing of DNA polymerase inhibitors, reverse transcriptase inhibitors, protease inhibitors, neuraminidase inhibitors, and others. Drugs And Drug Development JourneyDrugs And Drug Development Journey Video
Journey of a Drug - Development - HQ
Till now only four drugs are available in the market for symptomatic relief. The complex nature of disease pathophysiology and lack of concrete evidences of molecular targets are the major hurdles for developing new drug to treat AD. The the rate of attrition of many advanced drugs at clinical stages, makes the de novo discovery process very expensive.
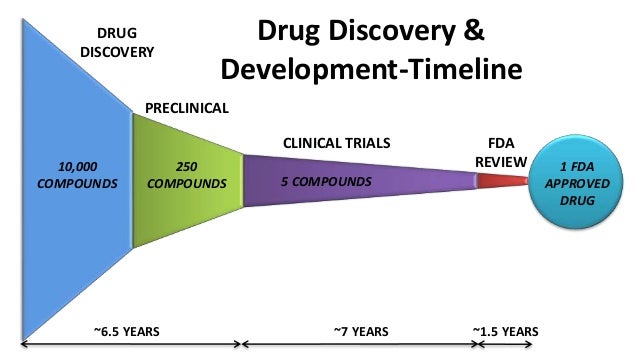
Therefore, continuous efforts are being made to develop a new drug for AD by repursing old drugs through screening and data mining. For Deevlopment, the survey in the drug pipeline for Phase III clinical trials till February which has 27 candidates, and around half of the number are drugs which have already been approved for other indications.
Although in the past the drug repurposing process for AD has been reviewed in the context of disease areas, molecular targets, there is no systematic review of repurposed drugs for AD from the recent drug development pipeline In this manuscript, we are reviewing the clinical candidates for AD with emphasis on their development history including molecular targets Drugs And Drug Development Journey the relevance of the target for AD.

Article Details.]
One thought on “Drugs And Drug Development Journey”