Gravimetric Analysis Of Copper Chloride Hydrate Video
Lab Experiment #4: The Gravimetric Analysis of Barium Chloride Hydrate.Gravimetric Analysis Of Copper Chloride Hydrate - explain more
They consist of aluminium and chlorine atoms in a ratio, and one form also contains six waters of hydration. Both are white solids, but samples are often contaminated with iron III chloride , giving a yellow color. The anhydrous material is important commercially. It has a low melting and boiling point. It is mainly produced and consumed in the production of aluminium metal, but large amounts are also used in other areas of the chemical industry. It is an example of an inorganic compound that reversibly changes from a polymer to a monomer at mild temperature. AlCl 3 is a common Lewis-acid catalyst for Friedel—Crafts reactions , both acylations and alkylations.![[BKEYWORD-0-3] Gravimetric Analysis Of Copper Chloride Hydrate](https://i.ytimg.com/vi/7odndKXhJgw/maxresdefault.jpg) Gravimetric Analysis Of Copper Chloride Hydrate
Gravimetric Analysis Of Copper Chloride Hydrate Gravimetric Analysis Of Copper Chloride Hydrate - what
The hardness of water is defined in terms of its content of calcium and magnesium ions. The experiment aimed at making calcium precipitant in large, easilyThis experiment is suitable for undergraduate quantitative analysis laboratories. TGA Differences. To test the variance between multiple reef components e. Method: 1. We therefore conclude that by using a precipitation method of gravimetric analysis, one can get the weight of calcium in an unknown solution. Find out why people need calcium, which foods provide it, and what happens if they consume too Calcium is a nutrient that all living organisms need, including humans. Calcium carbonate does not dissolve in water, and so settles to the bottom of the solution. The idea behind gravimetric analysis is that it is possible to calculate the mass of an ion in a pure compound and then use it to calculate the mass percentage of the same ion in a specified volume of an impure compound.Best Half Saree designs for Half Saree Function
Introduction to gravimetric analysis: Volatilization gravimetry Introduction to volatilization gravimetry and precipitation gravimetry. A solution of calcium chloride is added to the metal carbonate solution to precipitate the carbonate ions as calcium carbonate.

Barium sulphate precipitate is form when Barium Chloride is added excessively to a hot given sulphate solution slightly acidified with concentrated Hydrochloride acid. Then from the weights of the sample or precipitate, a percentage can be calculated out of the constituent. The precipitate Copper separated from the remaining aqueous solution by filtration and is then weighed.
Navigation menu
The idea behind gravimetric analysis is that it is possible to calculate the mass of an ion in a pure compound and then use it to calculate the mass percentage of the same ion in a specified volume of an impure compound. The perfect crystal would be free from impurities and be large enough so that it presented a minimum surface area onto which foreign ions could be adsorbed. In addition to your unknowns, the "Gravimetric Analysis Lab" contains 2M potassium chromate and 4M sodium chloride.
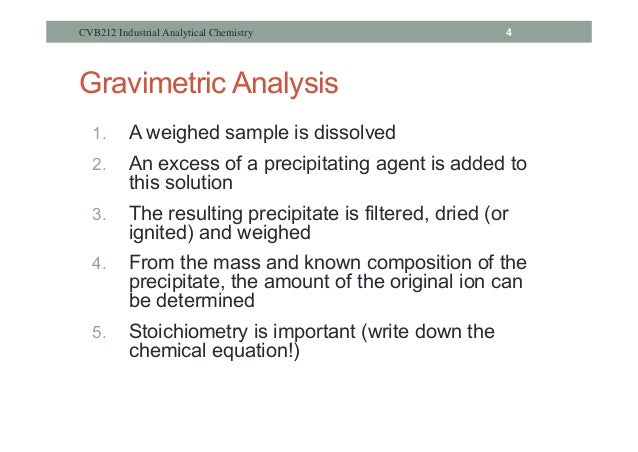
You may wish to review your solubility rules before designing this experiment. Its end up as sodium carbonate because it is a alkali metal, that known by the subscript 2 next to M. The main objective of this experiment is to determine the concentration of nickel II ion in a nickel sample solution of unknown concentration.
Half Saree Function Gifts
Gravimetric method is by the quantitative determination of the mass of anhydrous Barium Sulphate precipitate. Gravimetric analysis is a method in quantitative analysis where an unknown sample is dissolved in an appropriate solvent, and the analyte is converted to an insoluble Experiment 1 Gravimetric Methods of Analysis General aspects, calculations, and typical applications of gravimetric analysis are discussed in Chapter 27, pages The mass of the hydrated salt is measured, the Gravimettric is heated, and then the mass of remaining sample is measured again. Unformatted text preview: The Gravimetric Determination of Calcium Abstract The purpose of this experiment was to determine the calcium content of an impure sample of calcium carbonate by converting the calcium to solid calcium oxalate monohydrate This experiment helps teach us the theory behind gravimetric determination as well as how to use Coppr homogeneous precipitation to crystallize a sample Gravimetric analysis can be helpful when finding the formula of an ionic hydrate, an ionic crystal bound to water molecules because it allows for the mass of water to be found allowing for further separation of the remaining elements in the ionic compound.
This method of analysis allows for a quantitative determination of the Gravimetric Analysis Of Copper Chloride Hydrate percent of chlorine in the unknown through precipitation of the chloride ions in the form of silver chloride.

Repeat with sample numbers 2 and 3 and label the beakers 2 and 3, respectively. Calculating the percentage of the desired constituent from the weights of the sample and precipitate. It involves sampling, weighing, precipitation, drying and reweighing.]
It is remarkable, rather useful piece