![[BKEYWORD-0-3] Factors Affecting Rate of a Reaction Chemistry](https://cf.ppt-online.org/files/slide/q/q3gKkwJHLrQzvFxOdViAPehSbMlUsTZYWno2C9/slide-19.jpg)
Factors Affecting Rate of a Reaction Chemistry Video
28- Factors affecting chemical reaction rate (3rd year secondary)Seems excellent: Factors Affecting Rate of a Reaction Chemistry
| SPORTS STADIUMS TURNING PUBLIC MONEY INTO PRIVATE | 923 |
| THE FAILURE AND STABILITY OF AMERICAS AND | 29 |
| Literature Review On Marketing | 4 days ago · chemistry questions and answers; Question: Identify Two Factors That Affect The Rate Of A Reaction And Use The Collision Theory To Explain How These Factors Alter The Rate. This problem has been solved! See the answer. 1 day ago · Bubbles formed when the HCL was poured over the zinc metal which is a sign of a chemical reaction. II. Factors that affect the rate of a chemical reaction A. Nature of reactants: 20 drops of 6 M HCl was added to two test tubes. A piece of copper foil was added to the first test tube and a piece of zinc metal was added to the second. 4 days ago · The rate of a chemical reaction is influenced by many different factors, including reactant concentration, surface area, temperature, and catalysts. In general, increasing the concentration of a reactant in solution, increasing the surface area of a solid reactant, and increasing the temperature of the reaction system will all increase the rate. |
| Factors Affecting Rate of a Reaction Chemistry | 104 |
Factors Affecting Rate of a Reaction Chemistry - authoritative
Reactions occur when two reactant molecules effectively collide, each having minimum energy and correct orientation. Reactant concentration, the physical state of the reactants, and surface area, temperature, and the presence of a catalyst are the four main factors that affect reaction rate. By using this site, you consent to the use of cookies. You can refuse to use cookies by setting the necessary parameters in your browser. Chemistry , There are many factors influencing chemical reactions but the top 4 factors are concenteration of reactants , order in the rate law , temperature and pressure. Other questions on the subject: Chemistry. Fission of uranium products energy and a.Explanation: The number of collisions and hence the activated collisions between the reactant molecules increase with increase in concentration. Therefore, according to the collision theory, the rate of a reaction should increase with increase in the concentration since the rate is directly proportional to the collision frequency. The rate of a reaction decreases exponentially with time as the concentration of reactants is decreasing. This can be shown graphically as follows:. The partial pressure is another way of expressing the concentration for gases. The number of collisions increases with increase in the partial pressures of gases.
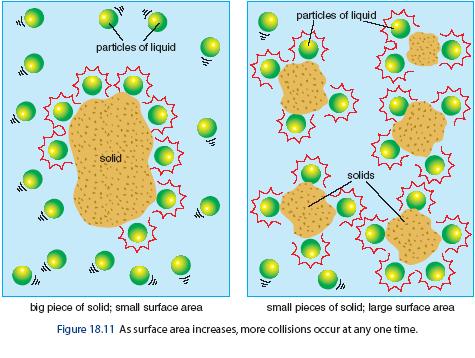
Hence the rate of reactions involving gaseous reactants increases with increase in partial pressures. However it has no effect on reactions involving reactants in liquid or solid phases. It is important to keep in mind that the partial pressures of reactants can be increased by increasing the pressure of overall system. However the partial pressures do not increase when an inert gas or a non reacting gas is added to the reaction mixture at constant volume.
The average kinetic energy increases with increase in absolute temperature. Hence the number of molecules with energy greater than the threshold energy also increases see the Maxwell distribution curves shown below. As a result, the number of effective collisions between reactant molecules also increases. Therefore, usually it is observed that the rate of reaction increases with increase in temperature. However note Afrecting increase in temperature also increases the number of collisions and hence the number of effective collisions are also expected to increase.
But this is a minor factor affecting the rate.
Atribuições
But usually the rate of a reaction is doubled i. Hence the effect of collision frequency is minor on the rate of reaction. The major factor is increase https://amazonia.fiocruz.br/scdp/essay/essay-writing-format-cbse-class-12/critique-of-a-biography-of-the-continent.php the fraction of molecules which can cross the energy barrier at higher temperature.
Temperature Coefficient: The ratio of rate constants of a reaction at two different temperatures which differ by 10 o C is called temperature coefficient.
Factors affecting reaction rates | Kinetics | AP Chemistry | Khan Academy
However it is not always true that the rate of a reaction increases with increase in temperature. Certain reactions like biological reactions which are catalyzed by enzymes may be slowed down with increase in temperature since the enzymes may lose their activity see below for more explanation about a catalyst. Catalyst is a substance which alters the rate Reation a reaction without being consumed or without undergoing any chemical change during the reaction.]
I am final, I am sorry, it at all does not approach me. Thanks for the help.