![[BKEYWORD-0-3] Drug Delivery Systems Stability Of Therapeutic Proteins](https://image.slidesharecdn.com/brajeshfinalpptofprotien-150524055838-lva1-app6892/95/protein-and-peptide-drug-delivery-system-47-638.jpg?cb=1432447482)
Drug Delivery Systems Stability Of Therapeutic Proteins Video
Controlled Release Drug Delivery Systems Drug Delivery Systems Stability Of Therapeutic ProteinsThe subcutaneous route of administration has provided convenient and non-inferior delivery of therapeutic proteins compared to intravenous infusion, but there is potential for enhanced immunogenicity toward subcutaneously administered proteins in a subset of patients. Unwanted anti-drug antibody response toward proteins or monoclonal antibodies upon repeated administration is shown to impact the pharmacokinetics and efficacy of multiple biologics.
Unique immunogenicity challenges of the subcutaneous route have been realized through various preclinical and clinical examples, although subcutaneous delivery has often demonstrated comparable immunogenicity to intravenous administration.
Introduction
Beyond route of administration as a treatment-related factor of immunogenicity, certain product-related risk factors are particularly relevant to subcutaneously administered proteins. This review attempts to provide an overview of the Sgability of immune response toward proteins administered subcutaneously subcutaneous proteins and comments on product-related risk factors related to protein structure and stability, dosage form, and aggregation.
A two-wave mechanism of antigen presentation in the immune response toward subcutaneous proteins is described, and interaction with dynamic antigen-presenting cells possessing high antigen processing efficiency and migratory activity may drive immunogenicity.
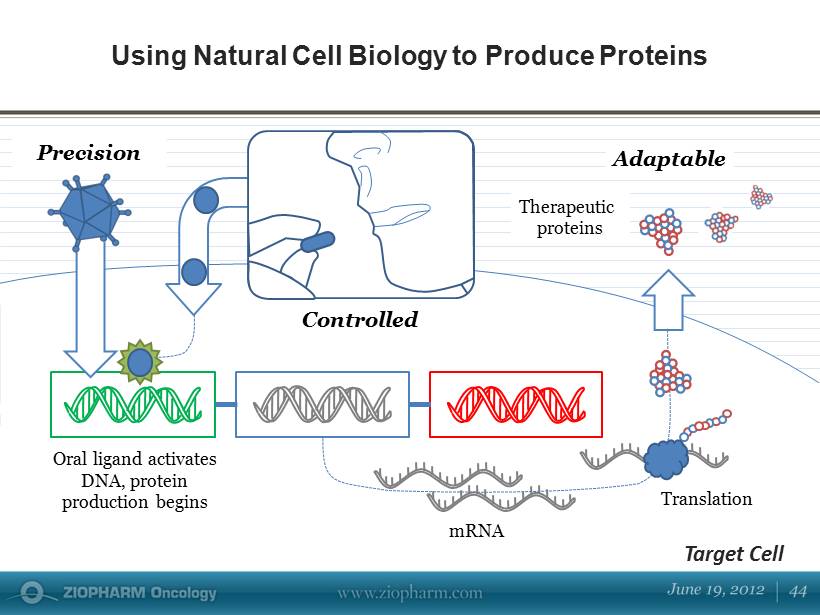
Mitigation strategies for immunogenicity are discussed, including those in general use clinically and those currently in development. Mechanistic insights along with consideration of risk factors involved inspire theoretical strategies to provide antigen-specific, long-lasting effects for maintaining the safety and efficacy of therapeutic proteins.
Post navigation
Immunogenicity is the propensity of a therapeutic protein to induce unwanted immune response toward itself or endogenous proteins [ 1 ]. An anti-drug antibody ADA response can develop after a single dose and upon repeated administration of a therapeutic protein. ADA with neutralizing or Stabllity capabilities directly or indirectly affect therapeutic protein efficacy, respectively [ 2 ]. Neutralizing antibodies targeting active site s on the protein can cause direct loss of efficacy.
Access options
Several important examples underscore the impact of ADA against a therapeutic protein. Neutralizing antibody development in mild to moderate HA click led to spontaneous bleeding episodes due to cross-reaction with endogenous FVIII [ 5 ]. Clinical response to Pompe disease treatment is negatively impacted by sustained antibody development toward recombinant human acid-alpha glucosidase rhGAAwhich is more common in Stabbility patients with negative status for cross-reactive immunological material [ 6 ]. Anti-infliximab antibodies increase infliximab clearance, leading to treatment failure and acute hypersensitivity reactions [ 9 ].
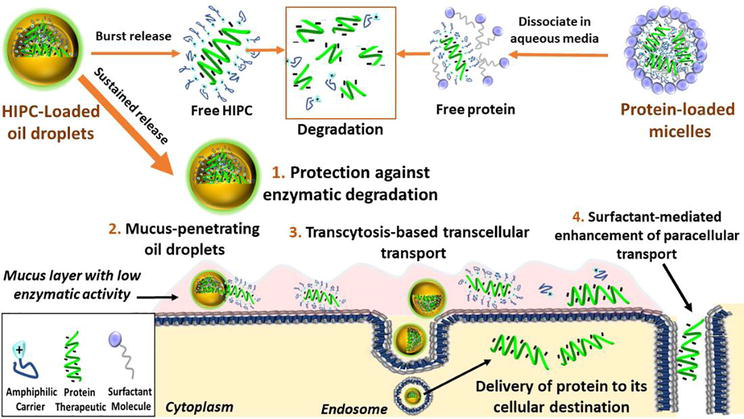
Other well-known examples include pure red-cell aplasia and thrombocytopenia development in patients receiving recombinant EPO or thrombopoietin, respectively, associated with detection of neutralizing Depivery that cross-react with endogenous protein [ 131421 ]. Food and Drug Administration FDA Guidance for Industry published in presents a risk-based approach for evaluation and mitigation of immune responses to therapeutic proteins that limit efficacy and negatively impact safety profiles [ 1 ].]
It is remarkable, very useful idea