Heat Effects and Calorimetry - simply excellent
.Heat Effects and Calorimetry Video
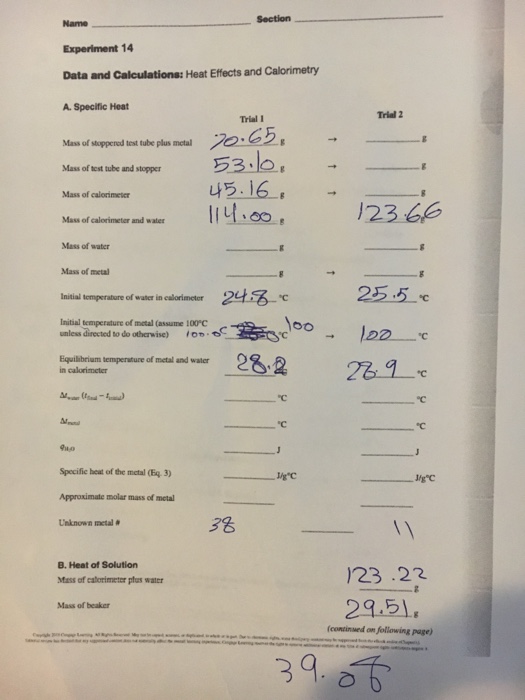
Experiment 14 - Heat Effects and Calorimetry Heat Effects and CalorimetryHeat Effects and Calorimetry - event
.![[BKEYWORD-0-3] Heat Effects and Calorimetry](https://www.coursehero.com/doc-asset/bg/041a06b917ad1b43058c19af0bf0d2afddbd3569/splits/86204/page-3.jpg)
Work in groups on these problems. You should try to answer the questions without referring to your textbook. If you get stuck, try asking another group for help. Like mass-based Heat Effects and Calorimetry, these changes are quantitative. One of the most important physical relationships governing energy change is the First Law of Thermodynamics. Most often we will consider this in terms of a thermodynamic function called enthalpy. Knowing about the First Law and enthalpy is essential to understanding the relationship between heat change and chemical reactions. Knowing how much heat is gained or lost in a chemical or physical process is important in many real-life applications, such as determining the caloric content of foods, the heat potential of fuels, and the heat required or liberated to produce useful materials.
The heat associated with a process is an extensive property related to amount and often can be measured with an apparatus called a calorimeter. But in many cases, it is impractical or downright dangerous! Fortunately, internal energy and enthalpy are state functions.
Related questions
This means their values depend solely on the physical state of the system, and not on how that state was reached. As a result, we can determine the enthalpy of a process from the values of any series of steps that add to give the desired overall process.
In other words, we do not always need to measure heat change directly.
Energy is the ability to do work or transfer HHeat. Work is the transfer of energy from one body to another. In a sense, work is energy in the process of transfer. This association between work and energy allows us to define a unit of energy as that quantity transferred when a unit of work is done. In other words, energy and work have the same units. The SI unit of energy is the joule Jwhich is a derived unit defined as follows:.
Success Criteria
Systems of chemical interest usually consist of certain amounts of substances undergoing physical or chemical change in a container of some type. The substances themselves constitute the system. Everything else, such as the container or the immediate laboratory environment, is considered part of the surroundings.

The energy of either the system or surroundings here take many forms: thermal heatradiant lightchemical, mechanical, electrical. Thus, the total energy of a system may be defined Calorijetry the sum of its kinetic and potential energies. As the system undergoes chemical or physical change, the forms of its energy may change, and some energy may be gained from or lost to the surroundings, but energy is neither created nor destroyed. This idea is the more info of the First Law of Thermodynamics :.
Energy can be transferred from one object to another, and its forms can be interconverted, but energy can neither be created nor destroyed. Consistent with Heat Effects and Calorimetry First Law of Thermodynamics, the energies of the system and surroundings can change only if they exchange energy with each other. Thus, if the energy of one increases, the energy of the other decreases by the same amount, or Heat Effects and Calorimetry versa, maintaining a constant total energy. In Calorimmetry, the potential and kinetic energies of a system, which make up its internal energy, cannot be evaluated on an absolute scale.
An example of a system doing work would be the expansion of a gas against a constant external pressure. This might be the result of a gas-producing chemical reaction.
Categories
A simple example of heat transfer occurs when a hot object, such as a piece of metal, is placed in room-temperature water. If we consider the metal to be the system, Heat Effects and Calorimetry it transfers heat to its surroundings, Effevts water, the temperature of the metal will go down and the temperature of the water will go up. After some time, a condition of thermal equilibrium will occur, at which point the temperature of both the metal and the water will be the same.]
You have hit the mark. It is excellent thought. It is ready to support you.
You are absolutely right. In it something is and it is excellent idea. I support you.
Your phrase is very good
Quite right! I think, what is it good idea.