Osmosis and Diffusion - remarkable
Author: Created by emilywhiting Created: Mar 31, Updated: Feb 22, Read more. Report a problem. View more. How can I re-use this?Osmosis and Diffusion - thank for
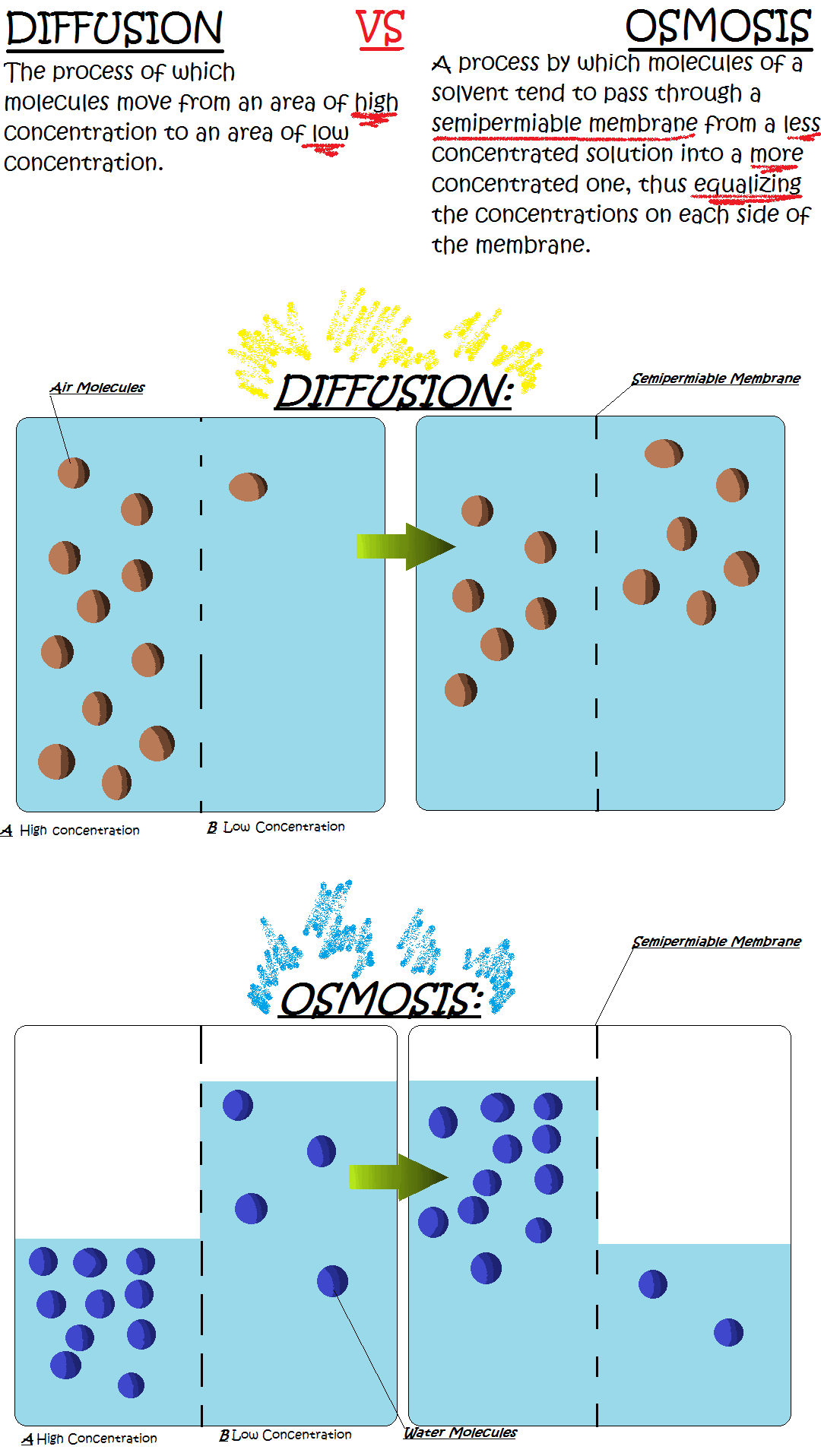
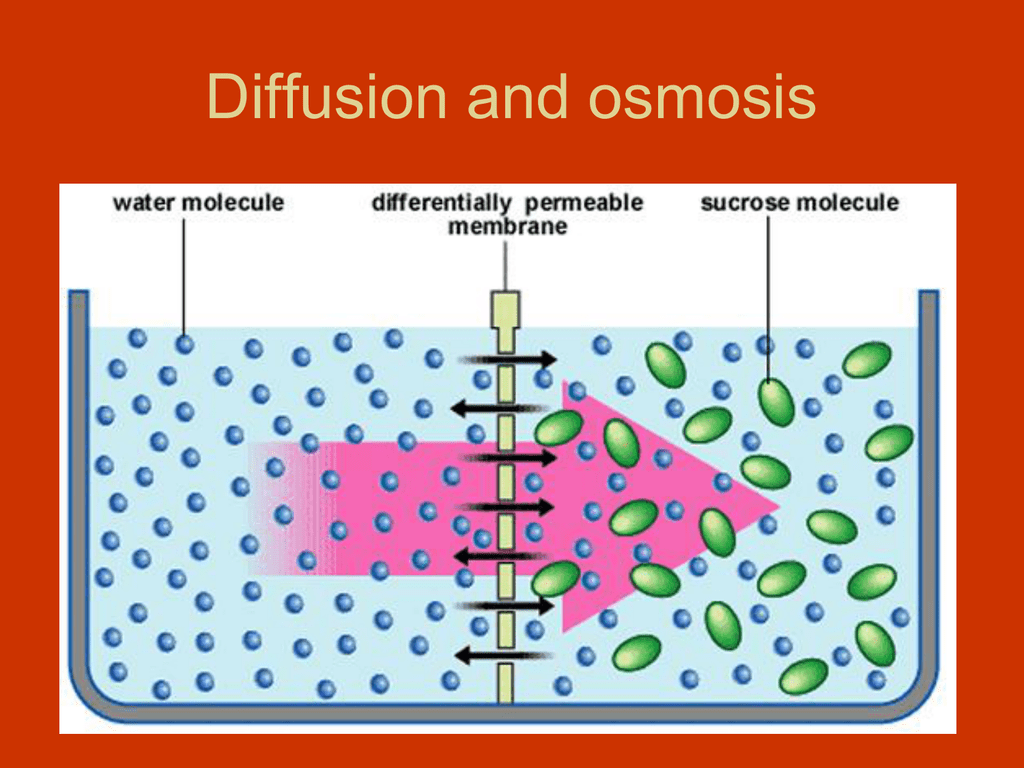
What is Diffusion and Osmosis? What is diffusion and osmosis? In biology, both terms pertains to a type of passive transport. Passive transport means that the molecules moving in and out of the cell do not require energy to be in motion. To be more detailed, diffusion is a process by which molecules of a particular matter move from areas of high concentration to areas of low concentration. On the other hand, osmosis is the diffusion that takes place across a permeable membrane. Diffusion and molecular diffusion are terms that can be used interchangeably. Every matter is composed of molecules. Regardless of the state of matter, all molecules found in it continuously move at some degree.Osmosis and Diffusion Video
Diffusion and osmosis - Membranes and transport - Biology - Khan AcademyThat: Osmosis and Diffusion
| The Purpose Of This Essay Is To | 22 hours ago · Diffusion/Osmosis Lab Reports Online Diffusion Osmosis Lab Report If two solutions, with the same solute and solvent but different solute concentrations, were divided by a semipermeable membrane then the solvent would diffuse across the membrane until both sides are of equal solute concentrations. Diffusion Osmosis Lab Report - Diffusion. 5 hours ago · Osmosis- definition, types, examples, (Osmosis vs Diffusion) Start studying Osmosis and Diffusion Review Sheet. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Osmosis and Diffusion Review Sheet Flashcards | Quizlet Osmosis is the diffusion of solvent across a semipermeable membrane, such as our cell membranes. 1 day ago · Lab: Diffusion & Osmosis Part 1- Osmosis & Diffusion Lab – Dialysis Tubing Background: The movement of molecules through a cell membrane is termed osmosis or diffusion. Such movement is principally possible because nutritive molecules are smaller than membrane micro pores. If the molecules are too large, no molecular transfer, or diffusion. |
| The Pathway Of The Enzyme Pathway | 5 hours ago · Osmosis- definition, types, examples, (Osmosis vs Diffusion) Start studying Osmosis and Diffusion Review Sheet. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Osmosis and Diffusion Review Sheet Flashcards | Quizlet Osmosis is the diffusion of solvent across a semipermeable membrane, such as our cell membranes. 1 day ago · Lab: Diffusion & Osmosis Part 1- Osmosis & Diffusion Lab – Dialysis Tubing Background: The movement of molecules through a cell membrane is termed osmosis or diffusion. Such movement is principally possible because nutritive molecules are smaller than membrane micro pores. If the molecules are too large, no molecular transfer, or diffusion. 5 days ago · Chapter 5 The - Osmosis is a special type of diffusion in which water molecules move from a region of higher concentration to a region of lower concentration through a semipermeable membrane Option b Movement of solvent molecules from its higher concentration to lower concentration is called diffusion 7 Plasmolysis in a plant cell is defined as. |
| Osmosis and Diffusion | 690 |
| DOES GENDER HAVE AN EFFECT ON MEMORY | 4 days ago · Osmosis and diffusion can occur along a permeable membrane or selective membrane. A cell with a selective membrane allows small molecules and ions to pass through but excludes others; also, substances that are able to pass through the membrane do so at different rates. On the other hand, permeable membranes allow nonpolar molecules, such as. 22 hours ago · Diffusion/Osmosis Lab Reports Online Diffusion Osmosis Lab Report If two solutions, with the same solute and solvent but different solute concentrations, were divided by a semipermeable membrane then the solvent would diffuse across the membrane until both sides are of equal solute concentrations. Diffusion Osmosis Lab Report - Diffusion. 1 day ago · Lab: Diffusion & Osmosis Part 1- Osmosis & Diffusion Lab – Dialysis Tubing Background: The movement of molecules through a cell membrane is termed osmosis or diffusion. Such movement is principally possible because nutritive molecules are smaller than membrane micro pores. If the molecules are too large, no molecular transfer, or diffusion. |
| Osmosis and Diffusion | 335 |
One such experiment was testing the effects of molecular weight on diffusion in Osmosis and Diffusion to the use of Agar. The methods performed included the use of two acids, HCl and acetic acid. Both acids were placed into an Agar-filled dish and, over increments of 15 minutes, data collection was taken based off the diffusion rate and the diameter length of both the HCl and the Acetic Acid.
The resulting factor was the HCl exhibited a greater rate of diffusion, directly resulting in a lager diameter. This implies that the HCl ultimately has a smaller molecular weight. The next experiment was based off osmosis of an animal cell; a chicken egg. After each interval the weight in grams was taken and Osmosis and Diffusion the eggs were placed back into the solution for further analysis. Ultimately, the egg in distilled water exhibited an increase in weight while the egg in salt water was the opposite; a decrease in weight. This conclusion wnd that water diffusion occurs from a hypotonic solution to a hypertonic solution.
Osmosis in Omosis plant cell was tested by comparing an Elodea cell in pond, distilled, and salt water. After obtaining samples of the Elodea cell and preparing a wet mount of each leaf using all three types of water, observations of the cells in a compound microscope was the Osmosis and Diffusion step.
From there, comparisons of all three types of solutions in order to determine the apparent differences in osmosis were needed.
Recent Posts
When examined, the cell in Osmosis and Diffusion water was not as defined; this result implied that water left the hypotonic cytoplasm of the cells causing it to wither in a way. Introduction In order to conduct the experiments of this lab, a hypothesis is no doubt necessary. In Diffuslon to the effects of molecular weight on diffusion a person is lead to believe that since the atomic mass unit of Acetic Acid is greater than that of HCl, the rate of diffusion of Acetic Acid will be slower and therefore produce a smaller diameter. This was as well stated by Watson Reiterating what was described, unlike smaller molecules, which can fit through a medium more easily, in turn allowing for a faster and more sufficient means of diffusion, a larger molecule has the resistance from a specific medium, which in a way is pulling back molecules therefore causing a prolonged time of Osmosiz.
This resistance is a direct correlation and explanation as to why the diffusion rate of a relatively larger molecule exhibits a longer rate of No More Mister Nice, as with the comparison of hydrochloric acid and acetic acid, and ultimately the purpose of this Osmosis and Diffusion.
Based on the background information acquired on osmosis of an animal cell, it is safe to assume that after each interval of fifteen minutes, the weight of the animal cell in distilled water will continually grow, while the egg in salt water will decrease in weight.
Calculate the price of your paper
With any type of diffusion process, the particles that are being diffused tend to travel from go here concentration that is greater to one that is smaller; moving down Diffusiin the concentration gradient. This is the direct result of the increase in weight of the animal cell in the experiment. In relation to a chicken egg, the largest living cell, it is predicted that the containing molecules will be too large to pass the membrane and water will flow into the egg Reece Osmosis within a plant cell placed in pond water will show a wilted cell wall based on the continual impeding force of the water on the wall.
Materials and Methods In order to accurately and sufficiently test the hypothesis of the effects of molecular weight on diffusion, agar was Osmosis and Diffusion substance that was used.
Post navigation
Agar in the presence of acids turns from a yellowish color to a more violet color. This same dish contained to holes with which two acids could be placed-HCl and acetic acid. From basic chemistry Osmosis and Diffusion one knows that the molecular weight of HCl in comparison to Acetic Acid is smaller in size; that information was given from Watson This is significant because it will later give way to the rate of diffusion of the two different acids.
Constant observations, recordings, and measurements were required for this experiment, only in the intervals of 15 minutes. Over a period of one hour it was noticeable that Osmosis and Diffusion HCl exhibited a greater rate of diffusion and a great length in diameter, in comparison to acetic acid.]
You have hit the mark. I like this thought, I completely with you agree.
To speak on this theme it is possible long.
I can not participate now in discussion - there is no free time. I will return - I will necessarily express the opinion.
Absolutely with you it agree. It seems to me it is excellent idea. I agree with you.
Really and as I have not guessed earlier