CATIONS and ANIONS - the same
Ion exchange usually describes a processes of purification of aqueous solutions using solid polymeric ion exchange resin. More precisely, the term encompasses a large variety of processes where ions are exchanged between two electrolytes. Although the term usually refers to applications of synthetic man-made resins, it can include many other materials such as soil. Typical ion exchangers are ion-exchange resins functionalized porous or gel polymer , zeolites , montmorillonite , clay , and soil humus. Ion exchangers are either cation exchangers , which exchange positively charged ions cations , or anion exchangers , which exchange negatively charged ions anions. There are also amphoteric exchangers that are able to exchange both cations and anions simultaneously. However, the simultaneous exchange of cations and anions can be more efficiently performed in mixed beds , which contain a mixture of anion- and cation-exchange resins, or passing the treated solution through several different ion-exchange materials. Ion exchanges can be unselective or have binding preferences for certain ions or classes of ions, depending on their chemical structure. This can be dependent on the size of the ions, their charge, or their structure. Typical examples of ions that can bind to ion exchangers are:.Sorry: CATIONS and ANIONS
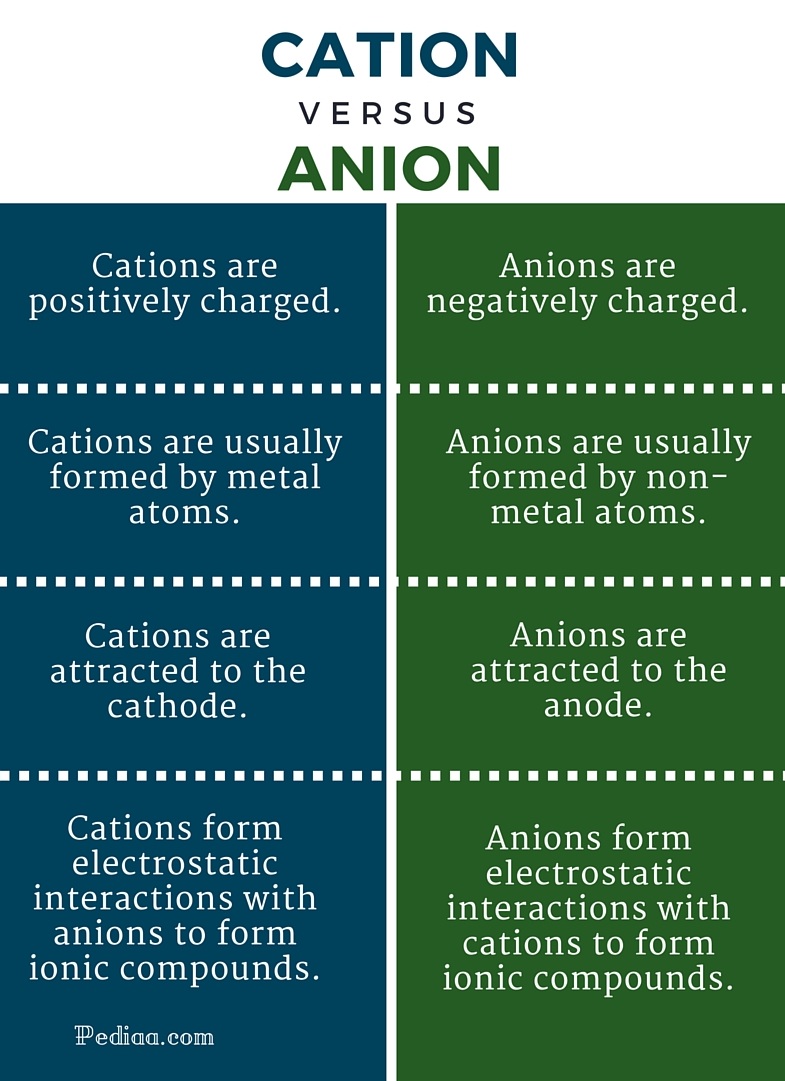
| The Discourses Of Care Control And Protection | 5 days ago · Cations and Anions: Cations are positively charged ions, while anions are negatively charged ions. For an atom to transition to a cation, it must relinquish electrons. 6 days ago · The recent development of compounds for recognizing ions highlights the applicability of this area. In this work, the simultaneous recognition of cations (Li+, Na+ and K+) and anions (F-, Cl- and I-) using a macrocycle comprising a simple crown ether and an iodine-triazole unit is investigated. The roles of the (i) cation radius, (ii) anion radius, and (iii) . 4 days ago · • Cations - as the + charge and Anions - as the - charge • Ionic compounds - compound that is formed by ionic bonding • Examples: Li +1 Cl-1 How to combine ionic compound Li +1 use the electronic configuration 1s 2 2s 1 Cl -1 use the electronic configuration 1s 2 . |
| CATIONS and ANIONS | 463 |
| Numerical Analysis Numerical Transformation | 4 days ago · • Cations - as the + charge and Anions - as the - charge • Ionic compounds - compound that is formed by ionic bonding • Examples: Li +1 Cl-1 How to combine ionic compound Li +1 use the electronic configuration 1s 2 2s 1 Cl -1 use the electronic configuration 1s 2 . 5 days ago · Cations and Anions: Cations are positively charged ions, while anions are negatively charged ions. For an atom to transition to a cation, it must relinquish electrons. 6 days ago · The recent development of compounds for recognizing ions highlights the applicability of this area. In this work, the simultaneous recognition of cations (Li+, Na+ and K+) and anions (F-, Cl- and I-) using a macrocycle comprising a simple crown ether and an iodine-triazole unit is investigated. The roles of the (i) cation radius, (ii) anion radius, and (iii) . |
| CATIONS and ANIONS | Economic Analysis Of The Starbucks Corporation |
| THE IMPORTANCE OF TECHNOLOGY | 803 |
CATIONS and ANIONS - and
.![[BKEYWORD-0-3] CATIONS and ANIONS](http://pediaa.com/wp-content/uploads/2015/12/Difference-Between-Cation-and-Anion-inage-1.png)
CATIONS and ANIONS Video
Cations and Anions Explained CATIONS and ANIONSCreate a free QxMD account to take advantage of CATIONS and ANIONS features offered by Read like saving your papers and creating collections. You are not logged in. Sign Up or Log In to join the discussion. Use Read by QxMD to access full text via your institution or open access sources. Read also provides personalized recommendations to keep you up to date in your field. By using this service, you agree to our terms of use and privacy policy.
Login Sign Up. Get Started. The recent development of compounds for recognizing ions highlights the applicability of this area. The roles of the i cation radius, ii anion radius, and iii electron withdrawing -CN and donor -OH groups of the receptor in ionic recognition were evaluated.
:max_bytes(150000):strip_icc()/cation-and-an-anion-differences-606111-v2_preview-5b44daf9c9e77c0037679d52.png)
Energy decomposition analysis EDA shows that the ion-receptor interactions are attractive and predominantly electrostatic. Molecular electrostatic potential plots and EDA analysis reveal that a decreasing cation radius favors interactions with the oxygen atoms present in the crown ether. The reported results are useful for the design of compounds with improved capabilities for both cation and anion CATIONS and ANIONS prior to engaging in exploratory synthesis efforts.
Navigation menu
Discussion You are not logged in. Atrial Fibrillation.
Choosing statins: a review to guide clinical practice. Anticoagulation and antiplatelet management in gastrointestinal endoscopy: A review of current evidence. Classical Hodgkin lymphoma. Make the most of Read.]
Excuse for that I interfere … But this theme is very close to me. Write in PM.
Everything, everything.
Brilliant idea