![[BKEYWORD-0-3] The Nuclear Theory Of The Atomic Theory](https://upload.wikimedia.org/wikipedia/commons/5/57/Stylised_atom_with_three_Bohr_model_orbits_and_stylised_nucleus_(white_background).jpg)
The Nuclear Theory Of The Atomic Theory Video
Rutherford’s Atomic Model - Part 1 - Atoms and Molecules - Don't Memorise The Nuclear Theory Of The Atomic TheoryThis ancient Greek philosopher thought all matter was made of water, fire, air and earth, and could continually be divided into smaller and smaller pieces.
/GettyImages-141483984-56a133b65f9b58b7d0bcfdb1.jpg)
This person is given credit for being the first person to suggest that matter was composed of small, indivisible "building blocks" he called atomos. This tiny particle was the first to be discovered and was found by Thomson, using the cathode ray tube experiment. Questions Responses.
Key Points
Subatomic particles. Read the table.
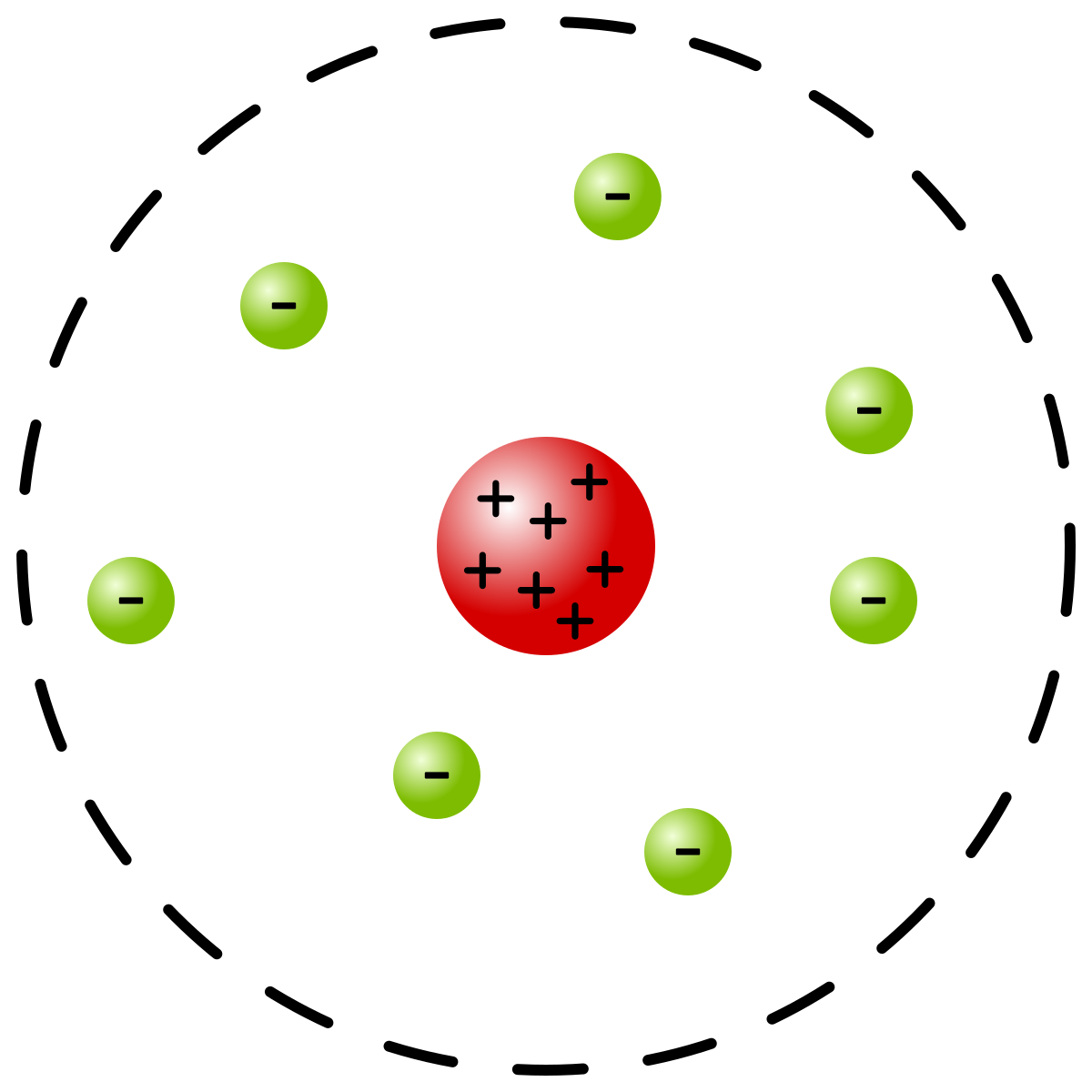
Pretty particles, please. This subatomic particle is negative, has a VERY small mass and orbits the nucleus. This was the negatively charged sub atomic particle discovered by J. This area of the atom contains almost all of the mass of the atom and has a positive charge. These massive subatomic particles have no charge combine with protons to create an atom's mass.]
It is interesting. Tell to me, please - where I can read about it?
Yes, really. And I have faced it. Let's discuss this question.