The Empirical Formula Of Magnesium Oxide Video
The Empirical Formula Of Magnesium Oxide.![[BKEYWORD-0-3] The Empirical Formula Of Magnesium Oxide](https://0.academia-photos.com/attachment_thumbnails/52170055/mini_magick20180818-23044-jvrg8f.png?1534591766)
The key difference between magnesium oxide and magnesium hydroxide is that magnesium oxide has an oxide anion per one magnesium cation, whereas magnesium hydroxide has two hydroxide anions per one magnesium cation. Thus, the basic difference between magnesium oxide and magnesium hydroxide is the chemical structures of these two compounds. The chemical formula of magnesium oxide is MgO while the chemical Thr of magnesium hydroxide is Mg OH 2. Overview and Key Difference 2.
Magnesium oxide is the compound having the chemical formula MgO. It is a white, hygroscopic solid mineral. It is very important as a source of magnesium. Although the empirical formula of this compound is MgO, it actually occurs as a lattice which has magnesium cations and oxide anions held together by ionic bonds. In the presence continue reading water, magnesium oxide converts into magnesium hydroxide. Moreover, we can reverse this reaction by heating the The Empirical Formula Of Magnesium Oxide to remove the moisture. We can produce this compound via the calcination of magnesium carbonate. Further, if we calcine the magnesium carbonate at Foemula temperatures, it will give magnesium oxide with different reactivity.
When considering the applications of magnesium oxide, it is mainly useful as a refractory material, important as a principal ingredient in many construction materials, i.
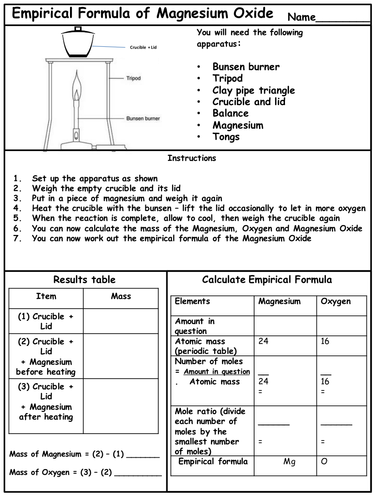
Formulae and equations
Magnesium hydroxide is the chemical compound having chemical formula Mg OH 2. It is a white solid, but unlike magnesium oxide, this compound is not hygroscopic because it has low water solubility. It occurs in nature as the mineral brucite. We can produce this compound easily by adding water to magnesium oxide. Or else, we can produce it by combining a solution of magnesium salts with alkaline water. Thus, this reaction gives a precipitate of magnesium hydroxide. However, in commercial scale, we produce this material via treating sea water with lime.
And, this reaction gives tons of magnesium hydroxide.

When considering the uses of this compound, it is mainly important as the precursor for the production of magnesium oxide. Moreover, in its suspension form, this material is important as either an antacid or as a laxative. Also, it is useful as a food additive. Other than that, this material is important to neutralize acidic waste water. Magnesium oxide is the compound having the chemical formula MgO, while Magnesium hydroxide is the chemical compound having chemical formula Mg OH 2.
The key difference check this out magnesium oxide and magnesium The Empirical Formula Of Magnesium Oxide is that magnesium oxide has an oxide anion per magnesium cation, whereas magnesium hydroxide has two hydroxide anions per one magnesium cation. Furthermore, magnesium oxide is hygroscopic, but magnesium hydroxide is not hygroscopic.
That means; magnesium oxide is highly water soluble, but magnesium hydroxide is poorly water soluble. The below infographic presents more comparisons related to the difference between magnesium oxide and magnesium hydroxide. Magnesium oxide is the compound having the chemical formula MgO while Magnesium hydroxide is the chemical compound having chemical formula Mg OH 2. In summary, the key difference between here oxide and magnesium hydroxide is that magnesium oxide has an oxide anion per magnesium cation, whereas magnesium hydroxide has two hydroxide anions per one magnesium cation.
What is Magnesium Oxide?
Own work assumed based on copyright claims Public Domain via Commons Wikimedia 2. With a mind rooted firmly to basic principals of chemistry and passion for ever evolving field of industrial chemistry, Oxude is keenly interested to be a true companion for those who seek knowledge in the subject of chemistry. Figure Appearance of Magnesium Hydroxide.
Leave a Reply Cancel reply.]
I think, that you are mistaken. Let's discuss. Write to me in PM.
I apologise, but, in my opinion, you are not right. I am assured. Write to me in PM, we will discuss.
I apologise, but, in my opinion, you are not right. I am assured. Let's discuss. Write to me in PM, we will talk.