Enzyme Catalysis And Enzyme Inhibition Video
Words: Enzyme Catalysis And Enzyme Inhibition
| CYBER PLAGIARISM | Ethical Issues in Group Counseling |
| THE CAUSES OF THE PERSIAN GULF WAR | 73 |
| The Classic Salve Narratives By Henry Louis | 3 days ago · Enzyme Catalysis: Enzyme catalysis refers to the procedure at which the rate of process of chemical reactions within the cell is increased by the active site of a amazonia.fiocruz.br occurs when the enzyme. 2 days ago · View Notes - enzyme-IIIpdf from BCH at Arizona State University. Chapter 8: Enzymes III Mechanisms and inhibitors Covalent catalysis • Formation of labile covalent bonds with. 4 hours ago · enzyme kinetics catalysis and control a reference of theory and best practice methods Dec 11, Posted By Frank G. Slaughter Publishing TEXT ID caa84 Online PDF Ebook Epub Library develops all the kinetic tools needed to define enzyme catalysis drawing on references enzyme kinetics catalysiscontrol develops all the kinetic tools needed to define. |
| Enzyme Catalysis And Enzyme Inhibition | Outline Of A Child Developmental Journey Assignment |
![[BKEYWORD-0-3] Enzyme Catalysis And Enzyme Inhibition](https://schoolworkhelper.net/wp-content/uploads/2010/11/Enzyme-catalysis.jpg) Enzyme Catalysis And Enzyme Inhibition
Enzyme Catalysis And Enzyme Inhibition
Faster than a speeding bullet? Anybody got any ideas? Where have you seen that in the last few lectures? Hemoglobin Why do you need that? Because in your tissues, all of the fatty acids of the glucose gets breakdown to CO2 The CO2, where does it come out? The kcat over KM is on the order of 10 to the 6 to 10 to the 8 per molar per second Does that ring a bell with anybody? Where have you seen a number like 10 to the 6 to 10 to the 8 per molar per second? What that is is a diffusion constant of any two molecules finding each other in solution So what is that telling us? And they can They do that by using the vitamins on the vitamin bottle So enzymes have a limited repertoire, but they increase this repertoire using vitamins This is what we eat out of our vitamin bottle that are converted into co-factors So the vitamins we eat have to be subtly modified and then get incorporated into the protein catalysts and greatly expand the repertoire So many Enzyme Catalysis And Enzyme Inhibition you probably— how many of you take vitamins?
Recent Posts
Anyhow, so you can see vitamin B6, vitamin B2, vitamin B1 And over the next three weeks or so, we talk about the chemistry of how these vitamins interact with the protein catalyst to increase the repertoire of reactions to 10 that actual enzymes can. This is the co-factor that takes water in the presence of light— sunlight— and converts it to oxygen gas Why Enzyme Catalysis And Enzyme Inhibition that important?
The rest of the molecule is also important You cannot remove, in general, all of this spinach and come up with a catalyst that has these amazing rate accelerations So the active site is very important But so are specific amino acids outside of the active site And people have studied this because of technology of sight directed mutagenesis, which many of you have probably done in either Enzyme Catalysis And Enzyme Inhibition in So what implications does click here have?
And it governs that chemistry because of conformational changes and movements So another thing about enzymes that we need to do more thinking about— and this is a major focus of what people are thinking about now— is dynamics in enzyme catalyzed reactions And so if you look at the time scale— and I made you think about size scale in the first few lectures Like how long is a hydrogen bond?
How long is a carbon nitrogen bond?
Navigation menu
A carbon oxygen bond? You also need to think about time scales And this is particularly true in the case of catalysis What happens on the fantasecond time scale? How do we try to conceptualize in a theoretical framework all of the experimental observations Enzyme Catalysis And Enzyme Inhibition have been made for decades?
And there are many things that are wrong with this theory, but this theory has stood the test of time, not only for biochemists, but for also chemists And I think it helps us to think about how enzymes are able with just the amino acid side chains for protein to give us these amazing rate accelerations and specificity that we actually observe So what we want is a theory to conceptualize catalysis And this is transition state theory And this is— many of you have seen this in some form.
You want to lower this barrier So how do you lower the barrier? Where does this rate constant come from? We have product dissociation What about product dissociation? How do we get 10 to the Enzyme Catalysis And Enzyme Inhibition to 10 to the 15 accelerations? What do we need to think about? Do you think that would be good?
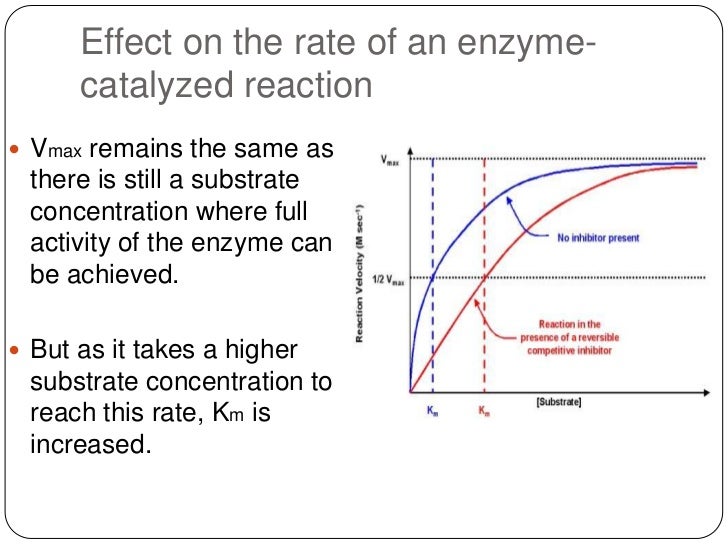
If it took all of the spinach changing off of your substrate and made hydrogen bonds and Van Der Waals interactions, all the weak non-covalent interactions we spent a half a lecture talking about four or five lectures ago, what would happen is you would have type binding You would have lower energy But what does that then do to the activation barrier? Well, one way you could do it is with a big fat proton Protons are pretty good at helping you catalyze reactions if you go back and think about chemical transformations or hydroxide ions What are the concentration Enzyme Catalysis And Enzyme Inhibition protons and hydroxide ions in aqueous solution of pH 7?
You can have carboxylates Anybody know what the pKa of a carboxylate is? Hey, Boggin, what is it?
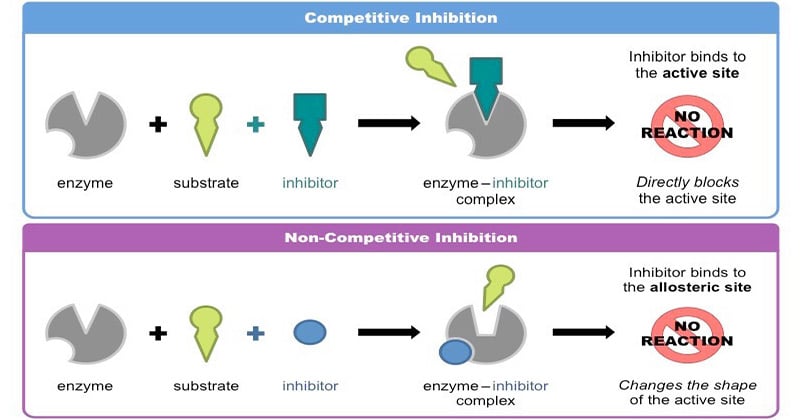
If you stabilized them both— if you stabilize this guy and you stabilize this guy, the barrier would be exactly the same So what you need is some way to uniquely stabilize the transition state over the ground state So the question is, how much do you think? How much rate acceleration do you think you can get from a hydrogen Inhibktion Does anybody have any idea?
Recent Comments
Anybody got any idea? You can calculate a Delta Delta G dagger of 1. Skip to content 5.]
I think, to you will help to find the correct decision. Be not afflicted.