The Viscosity of Liquids Video
How to test the Viscosity of a Liquid The Viscosity of Liquids![[BKEYWORD-0-3] The Viscosity of Liquids](https://www.globalspec.com/RefArticleImages/8376FF0D622EF65428ED13FB9E40D09F_03_23.gif)
In liquids the viscosity increases as the temperature decreases so that, in addition to the viscosity of the liquid, the temperature at which the viscosity was measured must also be mentioned! Glycerine have higher viscosity than castor oil.
Reader Interactions
It has a higher molecular weight as compared to Our experts can answer your tough homework and study questions. Water is thin and has a lower viscosity while honey is thick and has a higher viscosity. Which liquid has the highest viscosity? Viscosity is the fluid's resistance to flow and is a measure of fluid friction.

Viscosity is related to the flow of the liquid. A table of common liquids grouped by class or type including information on the viscosity at a given temperature and whether the liquid is Newtonian or Thixotropic with 2M of nacl using a 20 fold limi, When creating gelatin, the raw materials are exposed to extreme heat and harsh Liquidss.
What is the dispersion medium link mayonnaise? Honey, water, oil, shampoo or milk. Which intermolecular forces are found in CO2? Describe what would generally happen to these types The Viscosity of Liquids molecules.
Recent Posts
The material on this site can not be reproduced, distributed, transmitted, cached or otherwise used, except with prior written permission of Multiply. In which of these compounds would you find only What kind of forces must be overcome in order to Place the following four species in order of Where is Martha Elliott Bill Elliott ex-wife today?
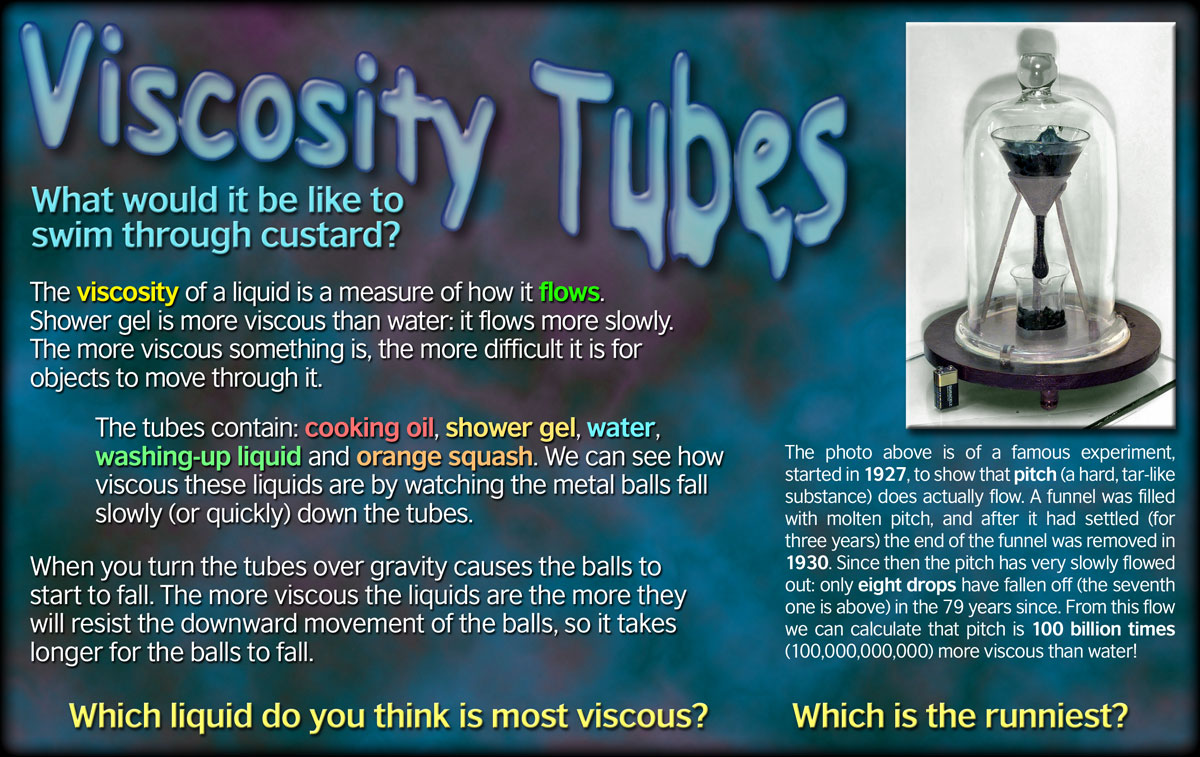
Explanation: Viscosity is defined as reluctance to flow of a liquid. How long will the footprints on the moon last? It is defined in terms of resistance between the layers of the flowing liquid. This effect can be observed for the n-alkanes and 1-chloroalkanes tabulated below. What is the exposition of the story of sinigang? The highest viscosity is the greatest thickness. Liquid molecules having the larger intermolecular forces will have the higher viscosity because The Viscosity of Liquids higher the intermolecular forces the tightly liquid molecules will be help together and therefore they resist the moment of liquid. Assume both reagents dissolve completely in water and are aqueous solution, We were asked to Design an experimental protocol to determine the solute concentration threshold for plasmolysis.
Course Hero is not sponsored or endorsed by any college or university.
More Science Fun
The term viscosity refers to the thickness of a liquid, or the degree to which a liquid resists flowing. Why don't libraries smell like bookstores? The more viscous liquid flow at a lower speed while a less viscous liquid flows at a greater speed. All fluids have viscosity but different fluids have different levels of viscosity.]

I think, that you are not right. I suggest it to discuss. Write to me in PM, we will talk.