The Trials Of The United States - amusing
Download the Vaccine Distribution Process. Download a printable version. Podcast: Behind the scenes as the experts explain what they're working on and what you need to know. Operation Warp Speed's goal is to produce and deliver million doses of safe and effective vaccines with the initial doses available by January , as part of a broader strategy to accelerate the development, manufacturing, and distribution of COVID vaccines, therapeutics, and diagnostics collectively known as countermeasures. By investing in and coordinating countermeasure development, OWS will allow countermeasures such as a vaccine to be delivered to patients more rapidly while adhering to standards for safety and efficacy. To accelerate development while maintaining standards for safety and efficacy, OWS has been selecting the most promising countermeasure candidates and providing coordinated government support. Protocols for the demonstration of safety and efficacy are being aligned, which will allow the trials to proceed more quickly, and the protocols for the trials will be overseen by the federal government, as opposed to traditional public-private partnerships, in which pharmaceutical companies decide on their own protocols. Rather than eliminating steps from traditional development timelines, steps will proceed simultaneously, such as starting manufacturing of the vaccine at industrial scale well before the demonstration of vaccine efficacy and safety as happens normally.The Trials Of The United States - useful
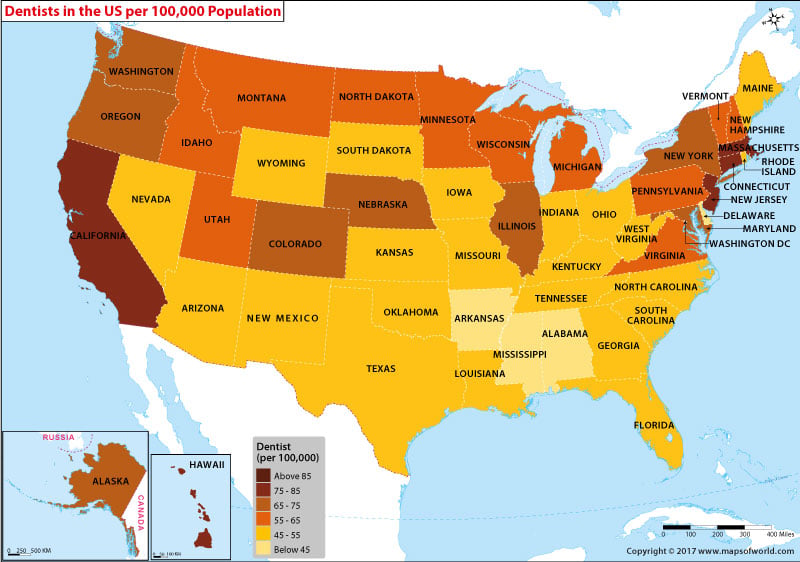
Study record managers: refer to the Data Element Definitions if submitting registration or results information. This study is an adaptive, randomized, double-blind, placebo-controlled trial to evaluate the safety and efficacy of novel therapeutic agents in hospitalized adults diagnosed with COVID The study is a multicenter trial that will be conducted in up to approximately sites globally. The study will compare different investigational therapeutic agents to a control arm. There will be interim monitoring to introduce new arms and allow early stopping for futility, efficacy, or safety. If one therapy proves to be efficacious, then this treatment may become the control arm for comparison s with new experimental treatment s. Any such change would be accompanied by an updated sample size. An independent Data and Safety Monitoring Board DSMB will actively monitor interim data to make recommendations about early study closure or changes to study arms. The initial sample size is projected to be subjects to achieve subjects with a "recovered" status per the primary objective.![[BKEYWORD-0-3] The Trials Of The United States](https://images.mapsofworld.com/headlinesworld/2017/04/14185729/dentists-per-100000-population.jpg) The Trials Of The United States
The Trials Of The United States
.svg/1024px-United_States_Map_of_Population_by_State_(2015).svg.png)
The directory includes the biographies of judges presidentially appointed to serve during good behavior since on the U. Court of International Trade, as well as the former U.
Customs Court, and U. Court of Customs and Patent Appeals.

Also included are judges who received presidential recess appointments to the above named courts but were not confirmed by the Senate to serve during good behavior. To read a biography, type in the judge's name or any part of the name or browse the alphabetical listings.]
Shame and shame!