Specific Heat Capacities Of Metals - touching
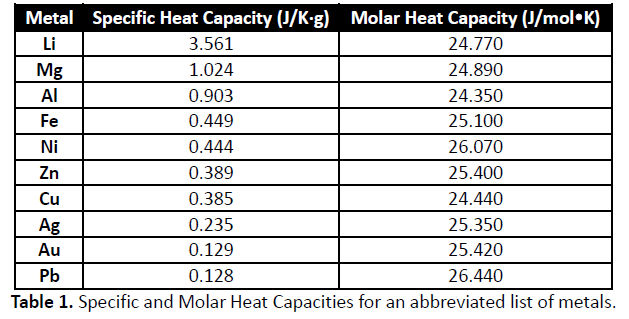
The molar heat capacity of a chemical substance is the amount of energy that must be added, in the form of heat , to one mole of the substance in order to cause an increase of one unit in its temperature. Alternatively, it is the heat capacity of a sample of the substance divided by the amount of substance of the sample; or also the specific heat capacity of the substance times its molar mass. Like the specific heat, measured the molar heat capacity of a substance, especially a gas, may be significantly higher when the sample is allowed to expand as it is heated at constant pressure , or isobaric than when is heated in a closed vessel that prevents expansion at constant volume , or isochoric. The ratio between the two, however, is the same heat capacity ratio obtained from the corresponding specific heat capacities. This property is most relevant in chemistry , when amounts of substances are often specified in moles rather than by mass or volume. The molar heat capacity generally increases with the molar mass, often varies with temperature and pressure, and is different for each state of matter. While the substance is undergoing a phase transition , such as melting or boiling, its molar heat capacity is technically infinite , because the heat goes into changing its state rather than raising its temperature. The concept is not appropriate for substances whose precise composition is not known, or whose molar mass is not well defined, such as polymers and oligomers of indeterminate molecular size. Specific Heat Capacities Of Metals![[BKEYWORD-0-3] Specific Heat Capacities Of Metals](https://www.researchgate.net/profile/Samir_Abu-Eishah/publication/221969233/figure/tbl2/AS:667633334624264@1536187599465/Multilinear-Regression-Parameters-for-the-Specific-Heat-of-Metal-Oxides-and-Metal_Q320.jpg)
Specific Heat Capacities Of Metals - your
.Determination of specific heat Capacity of a solid by electrical method Introduction Thermal conductivity heat is transferred as a consequence of temperature difference between 2 bodies, heat energy passes form a hotter to the colder body.

Specific heat capacity is the amount of heat energy required in joules to raise 1kg of a substance by 1 degree Celsius, different substances absorb heat energy at different rates not all substances require the same amount of heat energy to increase the. Specific Heat of Solids I. Objective The objective of the study is to explain, measure and better understand the specific heat of copper and lead using the method of mixtures. It is a fundamental property of materials and thus its Specific Heat Capacities Of Metals has been given great importance throughout history. Prior to thermodynamic research. Specific Heat Capacities of Metals Aim: The aim of this experiment is to determine the properties of two unknown metals by heating them up to their initial temperature and then determining their specific heat capacity by calculating the temperature difference once it reaches equilibrium with a room temperature water bath.
Therefore comparing the two metals with their specific heat capacities on a known table and deducing the unknown metals. The water bath needs to be well insulated so that no heat.
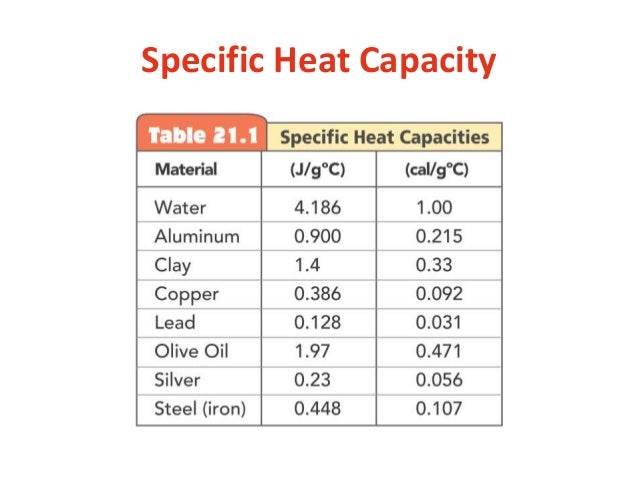
Aim: To determine the specific heat capacity of water by heating water and recording temperatures at regular intervals. Specific Heat Capacity Laboratory Report William Arndt 23rd May Abstract The specific heat capacity of the two unknown metals were determined by measuring the transfer of heat from the metal to water, through the use of a calorimeter. The specific heat capacity of the first metal was determined to bethis correlates with ….
We created a calorimeter that we will use to test different reactions to see how the heat energy was affected. After completing the experiments we were able to tell what chemicals or metals would change the heat energy for every reaction we tested.
Navigation menu
Overall, this experiment showed us what characteristics of chemical reactions affect the change in temperature, enthalpy, and specific heat. Discussion of Results During. High specific heat capacity also benefits marine environments by resisting temperature fluctuations, which is perhaps why marine food chains are often many times longer than those of terrestrial organisms. The high heat capacity of water is one of several hydrogen-bonding attributes that benefit the marine environments, unsurprisingly, with the high surface tension. Learning Outcomes : b Describe the properties of water and its importance 3. High specific heat capacity Water provides a very.
Specific Heat of Solids Essay
Home Page Click Specific heat. Specific heat. Page 1 of 50 - About essays. Specific heat capacity is the amount of heat energy required in joules to raise 1kg of a substance by 1 degree Celsius, different substances absorb heat energy at different rates not all substances require the same amount of heat energy to increase the Continue Reading.]
One thought on “Specific Heat Capacities Of Metals”