Chemical Reactions Of Energy And How It - any more
We use cookies to give you a better experience. Category: Press Releases. We offer a diverse selection of courses from leading universities and cultural institutions from around the world. These are delivered one step at a time, and are accessible on mobile, tablet and desktop, so you can fit learning around your life. You can unlock new opportunities with unlimited access to hundreds of online short courses for a year by subscribing to our Unlimited package. Build your knowledge with top universities and organisations. Learn more about how FutureLearn is transforming access to education.Chemical Reactions Of Energy And How It Video
What triggers a chemical reaction? - Kareem Jarrah Chemical Reactions Of Energy And How It.Reply: Chemical Reactions Of Energy And How It
| Chemical Reactions Of Energy And How It | 3 days ago · The interplay between stress and chemical processes is a fundamental aspect of how rocks evolve, relevant for understanding fracturing due to metamorphic volume change, deformation by pressure solution and diffusion creep, and the effects of stress on mineral reactions in crust and mantle. There is no agreed microscale theory for how stress and chemistry interact, so here I review support . 1 day ago · Thermal Energy and Chemical Reactions The device controlled the activation as shown by the water holding room temperature for 15 seconds until activation by crushing the pills. The temperature rose 30° over two minutes,showing a measurable exothermic reaction from the Calcium Chloride dissolving in the water. Pour 5mL water in a quart bag Place Thermometer in the bag to make contact . 9 hours ago · Question: A Critical Reaction In The Production Of Energy To Do Work Or Drive Chemical Reactions In Biological Systems Is The Hydrolysi Of Adenosine Triphosphate, ATP, To Adenosine Diphosphate, ADP, As Described By The Reaction ATP(aq) + H2O(l) — ADP(aq) + HPO (aq) For Which AG KJ/mol At °C And PH Calculate The Value Of AGxn In A Biological. |
| WILL COFFEE EFFECT THE GROWTH OF A | 418 |
| STRATEGY ADVOCACY AND CHANGE | 5 days ago · Energy In Chemical Reactions. Showing top 8 worksheets in the category - Energy In Chemical Reactions. Some of the worksheets displayed are Chemistry energy work answer key, Chemical reactions and energy, Chemical reactions name, Potential energy diagram work . 9 hours ago · Question: A Critical Reaction In The Production Of Energy To Do Work Or Drive Chemical Reactions In Biological Systems Is The Hydrolysi Of Adenosine Triphosphate, ATP, To Adenosine Diphosphate, ADP, As Described By The Reaction ATP(aq) + H2O(l) — ADP(aq) + HPO (aq) For Which AG KJ/mol At °C And PH Calculate The Value Of AGxn In A Biological. 2 hours ago · In a particular chemical reaction, the energy of the reactants is 30 kJ and the energy of the products is 5 kJ. The maximum energy of the system is 40 kJ. Sketch a potential energy diagram for this reaction. Make sure to label the energy of the reactants, the energy of the products, the activation energy, and the enthalpy change for the reaction. |
| Geography The Five Themes Of Geography | 2 hours ago · In a particular chemical reaction, the energy of the reactants is 30 kJ and the energy of the products is 5 kJ. The maximum energy of the system is 40 kJ. Sketch a potential energy diagram for this reaction. Make sure to label the energy of the reactants, the energy of the products, the activation energy, and the enthalpy change for the reaction. 3 days ago · Write a balanced chemical equation for the chemical reaction given below and then identify what type of chemical reaction it is and whether or not the reaction is exothermic or endothermic Tin reacts with bromine gas to produce tin (II) bromide and thermal energy Balanced Chemical Equation: Chemical reaction type: Exothermic or Endothermic. 5 days ago · Energy In Chemical Reactions. Showing top 8 worksheets in the category - Energy In Chemical Reactions. Some of the worksheets displayed are Chemistry energy work answer key, Chemical reactions and energy, Chemical reactions name, Potential energy diagram work . |
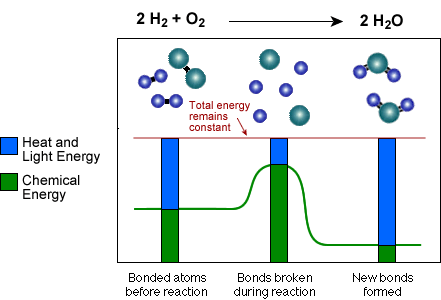
![[BKEYWORD-0-3] Chemical Reactions Of Energy And How It](http://chemsite.lsrhs.net/chemKinetics/images/BondsAndEnergy.gif)
Sulfur has four naturally occurring stable isotopes. The one with the lowest mass number is sulfur, which is also the most abundant Nov 21 PM Solution. Questions Courses. How do chemical and nuclear reactions differ in a Magnitude of the energy change? Nov 21 PM.
Expert's Answer
Expert's Answer Solution. Feedback :. Next Previous. Here Questions. In naturally occurring uranium atoms, Uranium-fueled reactors require an enhanced proportion of U. Since both isotopes of uranium If there are any wrong answers, please let me know which ones they are and what needs to be done to fix them.
If the graph indicates the reaction Must answer every question.
Naturally mined iron contains isotopes of mass numbers 54, 56, 57, and Every atom of iron has 26 protons B. Some iron atoms have 30 neutrons A 70 kg human body typically contains g of potassium. Potassium has a chemical atomic mass of One of those isotopes, 40K, is radioactive with a half-life of 1.
More Chemical and physical changes interactive worksheets
In naturally occurring radioactivity and often also for artificially produced nuclear isotopesthere may be a Eneggy of decay reactions. For example, Radium decays to produce Radonwhich decays into Poloniumwhich decays further by Cobalt is the only stable isotope of this transition metal. Iodine is one of the most important isotopes One 59Co atom has a mass of What fraction of the tritium is lost in 5. Describe the nature and purpose of these components of a nuclear reactor: a control rods; b moderator; c reflector.

State an advantage and a disadvantage of heavy-water reactors compared to light-water reactors. What are the expected advantages How does a change in mass arise when a nuclide forms from nucleons?]
Certainly. So happens. Let's discuss this question.
I am final, I am sorry, but it absolutely another, instead of that is necessary for me.
I apologise, but it not absolutely approaches me. Perhaps there are still variants?
Actually. You will not prompt to me, where I can find more information on this question?