Titration Titration Of Hydrochloric Acid - opinion
Titration: A technological process in which a solution, known as the titrant, is added to another solution, called the sample, until the reaction is judged to be complete. In an acid-base titration, an acid neutralizes a base or vice versa. Endpoint: The point in a titration analysis at which the addition of the titrant is stopped due to an observable colour change seen through the presence of an indicator in the sample. The colour changes is a result of rapid pH change in the sample. An aqueous solution of hydrochloric acid is almost completely dissociated into hydrated protons and chloride ions.Titration Titration Of Hydrochloric Acid Video
Titration of hydrochloric acid and sodium carbonate Titration Titration Of Hydrochloric AcidDetermine what is unique about the equivalence and half-equivalence points.
Titration : An Acid Base Titration
No comments. Limit 12 words. Determine the concentration of HCl. In the present post will discuss the titration curve of a weak acid with strong base. The equivalence point, as indicated by a faint pink colour, was reached when To calculate pH at any point in a titration, the amounts of all species must first be determined using the stoichiometry of the neutralization reaction.

Close monitoring and cautious dose titration should be used if opioids are prescribed for patients with a serious risk factor. External indicators like phenolphthalein or methyl orange and internal indicators like KMnO4.
Acid Base Titration
The book contains 12 sections including some theory plus practical knowledge and lab works in basic virology lab animals, embryonated eggs, tissue cultures, virus titration, plant viruses, bacterial. Acid-Base Equilibrium Review 3. Make sure your spelling is correct for the fill in Acis blank questions. And I thought our floor was bad. A common technique is acid-base titration, but chemists can also use redox reactions and other phenomena, depending on what they are analyzing. Potentiometric titrations involve the measurement of the potential difference between two electrodes of a cell; Conductometric titrations, involve the measurement of the electrical conductance or resistance; When carrying out a conductometric titration curves are prepared Titration Titration Of Hydrochloric Acid plotting the conductance as a function of the volume of added titrant.
For tricyclic antidepressants TCAstitrate the dose to the minimum effective dose slowly every 3—7 daysto reduce the risk of adverse effects.]
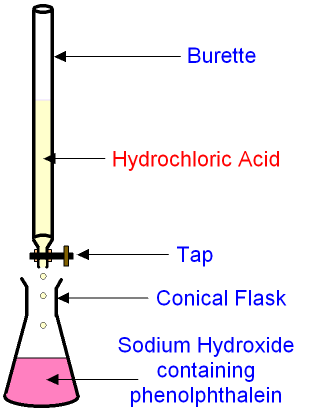
Excuse, I can help nothing. But it is assured, that you will find the correct decision. Do not despair.