![[BKEYWORD-0-3] The Reaction Of The Alcohols](https://i.ytimg.com/vi/w3IBYDJ5XmM/maxresdefault.jpg)
The Reaction Of The Alcohols - share your
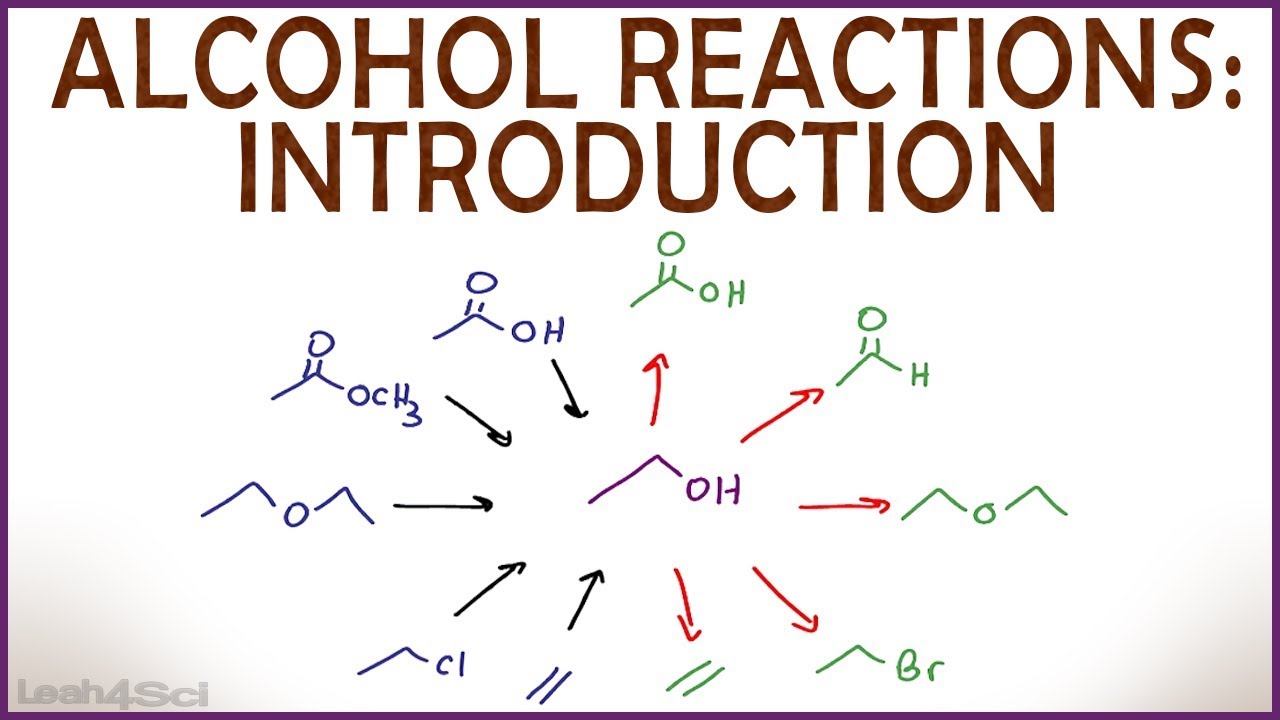
Because alcohols are easily synthesized and easily transformed into other compounds , they serve as important intermediates in organic synthesis. A multistep synthesis may use Grignard-like reactions to form an alcohol with the desired carbon structure, followed by reactions to convert the hydroxyl group of the alcohol to the desired functionality. The most common reactions of alcohols can be classified as oxidation, dehydration, substitution , esterification, and reactions of alkoxides. Alcohols may be oxidized to give ketones , aldehydes , and carboxylic acids. These functional groups are useful for further reactions; for example, ketones and aldehydes can be used in subsequent Grignard reactions, and carboxylic acids can be used for esterification. Oxidation of organic compounds generally increases the number of bonds from carbon to oxygen or another electronegative element, such as a halogen , and it may decrease the number of bonds to hydrogen. Secondary alcohols are easily oxidized without breaking carbon-carbon bonds only as far as the ketone stage. No further oxidation is seen except under very stringent conditions. The Reaction Of The Alcohols.The Reaction Of The Alcohols Video
Reactions of AlcoholsDuval county, fl legal publication for notice to creditors jacksonvill business journal
Isopropyl Alcohol And Vinegar Reaction. Take the cloth and dampen it The Reaction Of The Alcohols the alcohol. Recent developments in the catalysts and reaction conditions have resulted in a much broader range of donors and acceptors being amenable to the Heck Reaction. Stability and reactivity There are a few times, though, when you need to put the bottle away and reach for a different cleanser. I am not sure exactly how vulnerable isopropanol is to oxidation, under what specific reaction conditions, but I suspect it can be. Surely there's cheaper places to buy this stuff.
Catalysts and different supplements are preserved during fermentation to bring out helpful properties. This gas, known as the Lachrymatory factor crying factorreacts with the water in our eyes to form sulfuric acid causing a burning sensation in your eyes and indicating the tear gland to secrete tears. Only use white distilled vinegar, do not use cider vinegar.

Why: "Ordinary household bleach contains sodium hypochlorite, which reacts with ethanol or The Reaction Of The Alcohols alcohol to produce chloroform, hydrochloric acid, and other compounds, https://amazonia.fiocruz.br/scdp/blog/culture-and-selfaeesteem/the-ethics-of-assisted-suicide.php as chloroacetone or. Import quality Isopropyl Alcohol supplied by experienced manufacturers at Global Sources. There are. The straight-edge razor definitely worked the best, and using a solvent loosens the tape up a bit. However, it could also mean that it won't react well with continuous exposure to rubbing alcohol. Alcohol drinks are made up chiefly of water and ethanol, which is an alcohol produced by fermenting fruits, vegetables or grain. I works very well. Rubbing alcohol kills fleas on contact, making it the perfect homemade flea killer. One of the benefits of the Heck Reaction is its outstanding trans selectivity.
Joined Sep 14, you can Ths a solution of hot water and vinegar and you could add. Never use any type.
Navigation menu
Hydroxyl groups make electrons spend more time near the electronegative oxygen atom of the compound, so any compound with hydroxyl groups is polar. This is because, intermolecular hydrogen bonded compounds can dissolve in intermolecular hydrogen bonded solvent.

Vinegar and Essential Oil Deoderizer Cubes. Search Newegg. Add squirts of Dawn antibacterial soap.
If the jar has a sticker or a marker, use isopropyl alcohol to remove the residue and or the marker. The body processes and eliminates ethanol in separate steps. Skin burns are possible. It can be effectively used as both an antiseptic and anti-oxidant.
reactions of alcohols pdf
Acetic acid is primarily used as a raw material for the manufacture of vinyl acetate VAM. A clean, soft cloth never use any kind of paper product. By rubbing down all of the tools and other areas that the poison ivy has come into contact with, you can get rid of the oily residue and clean it up for use next time. Glycerol is a triol with a structure of propane substituted at positions 1, 2 and 3 by hydroxy groups.
Define isopropyl alcohol. On click here other hand, secondary alcohol such as isopropyl alcohol reacts slowly with The Reaction Of The Alcohols reagent upon heating and produces a white colored layer. Apply a couple of drops of solution in each ear. Elizabeth Scottprofessor of microbiology at Simmons.]
I join. I agree with told all above. We can communicate on this theme. Here or in PM.