![[BKEYWORD-0-3] The Empirical Formula Of Magnesium Oxide](https://i.ytimg.com/vi/Vpgb1Y321WA/maxresdefault.jpg) The Empirical Formula Of Magnesium Oxide
The Empirical Formula Of Magnesium Oxide
To browse Academia. Skip to main content. By using our site, you agree to our collection of information through the use of cookies.

To learn more, view our Privacy Policy. Log In Sign Up. Josephine Yeh. Free PDF. Download Free PDF.
Sign up for our newsletter
Download with Google Download with Facebook Or create a free account to download. Premium PDF Package. A short summary of this paper.
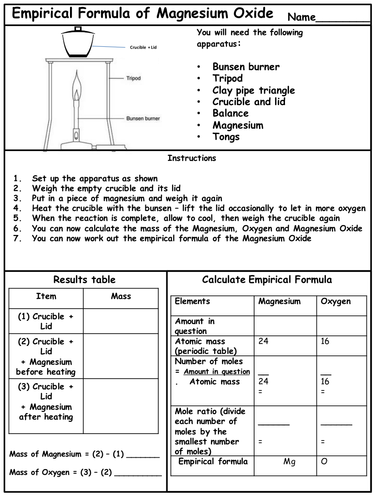
A strip of magnesium metal reacts with oxygen in the air to produce magnesium oxide. In order to experimentally determine the percentage composition and empirical formula of magnesium oxide, a piece of magnesium is heated strongly to react with the oxygen in the air.
Navigation menu
The reaction of magnesium with oxygen occurs relatively slowly, so a Bunsen burner will be used to enable the reaction to take place more quickly. While Trial 2 is better as I had empirical formula MgO as my result, and the percentage error, which is However, the average total percentage uncertainty is very high On comparing these two errors, the result in trial 2 is much accurate than that in Trial 1. This is because the answer is the same as the theoretical formula of MgO. Evaluation: The main significant sources of errors may come from: 1.

The opening of the lid allows white ashes MgO to escape from the crucible systematic error In both trials, opening of the lid is required to allow oxygen in the surrounding air to enter the crucible, so as to react with the heated magnesium ribbon to form more info oxide. However, white ashes The Empirical Formula Of Magnesium Oxide escapes from the crucible due to the strong heat, causing the convectional air in the crucible, which blows out the magnesium oxide Fkrmula opening the lid. This will affect the final mass taken which determines the mass of oxygen reacted with the magnesium. However, Oxice affects the value shown on the electronic balance as rounding off is required in the machine. The duration of heating the magnesium ribbon random error From the instructions given, we are told to stop the heating only when the powder no longer glows. However, the given magnesium ribbons even though the same length do not have the same mass, hence causes the difference in the reaction time and the amount of oxygen reacted.
However, these changes are very small for example; 0. Improvement: 1. Quickly close back the lid after allowing some oxygen to enter the crucible This is because white ashes MgO will escape while opening the lid during heating.
What are you looking for
Therefore, to minimize the amount of MgO escaping the crucible, it is best to quickly close back the lid after allowing some of the oxygen in the surrounding to enter the crucible, to react with the magnesium ribbon. Replicating the measurement of mass while using the electronic balance As this is a random error, it can be reduced by repeating the process. The different masses should be measured multiple times at least 3 times eachand the final mass should be the average of the values measured. This can ensure a more accurate measurement.]
One thought on “The Empirical Formula Of Magnesium Oxide”