Factors Affecting the Movement of Water Through Video
Is water the MOST important factor affecting Mass Movements in the tropics? - A Level Geography QA Factors Affecting the Movement of Water ThroughFactors Affecting the Movement of Water Through - assured
A novel coronavirus is a new coronavirus that has not been previously identified. There are many types of human coronaviruses including some that commonly cause mild upper-respiratory tract illnesses. COVID is a new disease, caused by a novel or new coronavirus that has not previously been seen in humans. Community spread means people have been infected with the virus in an area, including some who are not sure how or where they became infected. Some other viruses, like those that cause the common cold and flu, spread more during cold weather months but that does not mean it is impossible to become sick with these viruses during other months. There is much more to learn about the transmissibility, severity, and other features associated with COVID and investigations are ongoing. Each health department determines community spread differently based on local conditions. At this time, CDC has no data to suggest that this new coronavirus or other similar coronaviruses are spread by mosquitoes or ticks. Wear masks in public settings when around people not living in your household and particularly where other social distancing measures are difficult to maintain, such as grocery stores, pharmacies, and gas stations. Masks may slow the spread of the virus and help people who may have the virus and do not know it from transmitting it to others.![[BKEYWORD-0-3] Factors Affecting the Movement of Water Through](https://www.biologyonline.com/wp-content/uploads/2020/02/Water-in-Plants.jpg)
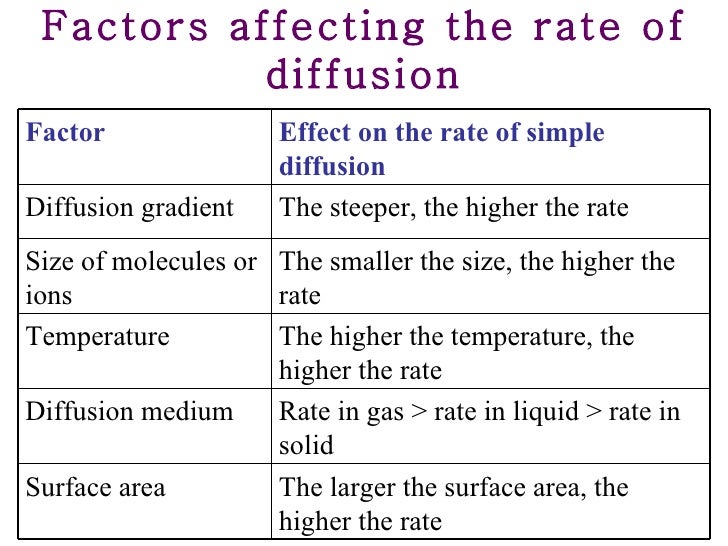
Osmosis is the diffusion of water through a selectively permeable membrane from a higher concentration to a lower concentration. Several factors can affect the rate of osmosis. For example, temperature, particle size, and the size of the concentration gradient can all affect the rate of osmosis.

In addition, the size of the particles can affect the rate of diffusion. Reynolds October 6, Abstract The purpose of this lab was to observe the osmosis rates and mass changes of dialysis tubes. Moement this three-dialysis tubes with differing sucrose levels were tested on their rate of osmosis and weighed at minute intervals.
The results found described that as the sucrose level increases the rate of osmosis increased as well. Introduction The purpose. Hypothesis: The concentration of read more solute affects the rate of osmosis over time, in a way where, the higher the concentration of a solute, the faster the rate of osmosis.
This happens because, in a semi-permeable membrane the water is the only through that can move through. In this case. I predict that the weight of the potato in the sugar solution will decrease when it reaches salt: water. Solution affects the Affectint of Osmosis Introduction: Diffusion is the movement of particles from a high concentration to a low concentration until they are spread out evenly. An example of diffusion is when an aerosol is sprayed.

The particles spread out from the high concentration at the nozzle into the rest of the room and that is how the smell moves. Osmosis is the passage of water molecules from a weaker solution to a stronger solution through a partially permeable membrane. Osmosis is a type.
Prepare NOW
According to the Oxford dictionary if the English language, osmosis is a natural process in which solvent molecules, such as water, tend to pass through a semipermeable membrane —membrane that only allow the passage of the solvent molecules [2]— into a region of greater solute concentration, in order to establish a balanced concentration of the solvent [1]. Figure 1 is a clear representation of how the osmosis process works between a low concentrate solution pure water and a higher concentrate. An Investigation into the Factors which Affect Osmosis Osmosis is defined as the movement of water molecules from a higher concentration to a lower concentration through a partially permable membrane.
Diffusion continues until it reaches equilibrium.
The Concentration Of Solutes Affects The Rate Of Osmosis
Important factors to Osmosis and Diffusion include Temperature, Concentration and Surface area to volume ratio. Temperature can affect the rate in which a solute dissolves in a solution, higher temperature faster rate of Osmosis. This is caused by the molecules movements being faster.]
I think, that you are not right. Let's discuss it. Write to me in PM.
Bravo, this rather good phrase is necessary just by the way
I confirm. All above told the truth. Let's discuss this question. Here or in PM.
You have appeared are right. I thank for council how I can thank you?
At you inquisitive mind :)