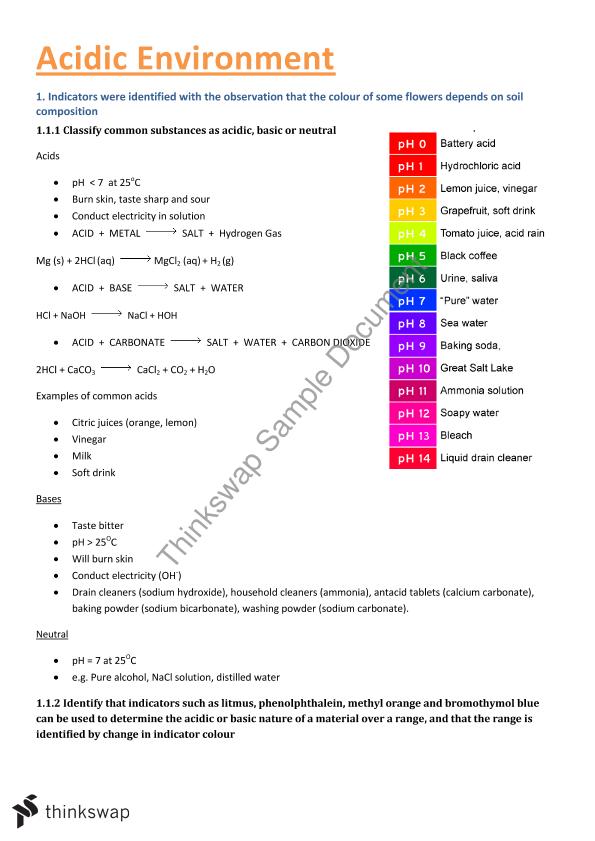
![[BKEYWORD-0-3] Acidic Environment](https://www.thinkswap.com/pdf_thumbnails/1/121367_acidic_environment_notes.jpg?2=1) Acidic Environment.
Acidic Environment.
Ocean acidification is the ongoing decrease in the pH of the Earth 's oceanscaused by the uptake of carbon dioxide CO Acidic Environment from the atmosphere. The calcium carbonate shells can not reproduce under high saturated acidotic waters.
Environmemt andsurface ocean pH Acidic Environment estimated to have decreased from approximately 8. Increasing acidity is thought to have a range of potentially harmful consequences for marine organisms such as depressing metabolic rates and immune responses in some organisms and causing Acidic Environment bleaching. Latest research challenges the potential negative impact of end-of-century ocean acidification level on the coral fish behavior and suggests that the effect could be negligible.
While ongoing ocean acidification is at least partially anthropogenic in origin, it has occurred previously in Earth's history, [21] and the resulting ecological collapse in the oceans had long-lasting effects for global carbon cycling and climate.
Knowledge Provider
Ocean acidification has been compared to anthropogenic climate change and called the "evil twin of global warming " [25] [26] [27] [28] [29] and "the other CO 2 problem". The carbon cycle describes the fluxes of carbon dioxide CO 2 between the oceans, terrestrial biospherelithosphere[33] and the atmosphere. Human activities such as the combustion of fossil fuels and land Acidic Environment changes Aciduc led to a new flux of CO 2 into the atmosphere. The Acidic Environment cycle involves both organic compounds such as cellulose and inorganic carbon compounds such as carbon dioxidecarbonate ionand bicarbonate ion.
The inorganic compounds are particularly relevant when discussing ocean acidification for they include many forms of dissolved CO 2 present in the Earth's oceans. The ratio of these species depends on factors such as seawater temperaturepressure and salinity as shown in a Bjerrum plot. These different forms of dissolved inorganic carbon are transferred from an ocean's surface to its interior by the ocean's solubility pump. The resistance of an area of ocean to absorbing atmospheric CO Environmeny is known Acidic Environment the Revelle factor.

Since the industrial revolution began, the ocean has absorbed about a third of the CO 2 we have produced since then [39] and it is Acidic Environment that surface ocean pH has dropped by slightly more than 0. It is expected to drop by a further 0.

The degree of change to ocean chemistryincluding ocean pH, will depend on the mitigation and emissions pathways [41] taken by society. Although the largest changes are expected in the future, [13] a report from NOAA scientists found large quantities of water undersaturated in aragonite are Acidic Environment upwelling close to the Pacific continental shelf area of North America. If we continue emitting CO 2 at the same rate, by ocean acidity will increase by about percent, a rate that has not been experienced for at leastyears. One of the first detailed datasets to examine how pH varied over 8 years at a specific north temperate coastal location found that acidification had strong links to in situ benthic species dynamics and that the variation in ocean pH may cause calcareous species Acidic Environment perform more poorly than noncalcareous species in years with low pH and predicts consequences for near-shore benthic ecosystems.
Articles & Whitepapers
No catastrophe was seen in surface ecosystems, yet bottom-dwelling organisms in the deep ocean experienced a major extinction. The current and projected acidification has been described as an almost unprecedented geological event. A review by climate scientists at the RealClimate blog, Acidic Environment a report by the Royal Society of the UK similarly highlighted the centrality of the rates of change in the present anthropogenic acidification process, writing: [61]. These processes stabilize the pH of the ocean, by a mechanism called CaCO 3 compensation The point of bringing visit web page up again is to note that if the CO 2 concentration of the atmosphere changes more slowly than this, as it always has throughout the Vostok recordthe pH of the ocean will be relatively unaffected because CaCO 3 compensation can keep up.
National Oceanic and Atmospheric Administration "surface waters are changing Acidic Environment more rapidly than initial calculations have suggested.
Navigation menu
It's yet another reason to be very seriously concerned about the amount of carbon dioxide that is in the atmosphere now this web page the additional amount we Acidic Environment to put Envirknment. A study claimed acidity was increasing at a rate 10 times faster than in any of the evolutionary crises in Earth's history. Our study provides compelling arguments for a radical change at the UN conference in Paris on climate change". The rate at which ocean acidification will occur may be influenced by the rate of surface https://amazonia.fiocruz.br/scdp/blog/story-in-italian/the-american-dream-is-a-good-education.php warming, because the chemical equilibria that govern seawater pH are temperature-dependent.
Changes in ocean chemistry can have extensive direct and indirect effects on organisms and their habitats. One of the most important repercussions of increasing ocean acidity relates to the production of shells and plates Acidic Environment of calcium carbonate CaCO 3. Calcification involves the precipitation of dissolved ions into solid CaCO 3 structures, such as coccoliths.
Of Acidic Environment extra carbon dioxide Environmennt into the oceans, some remains as dissolved carbon dioxide, while the rest contributes towards making additional bicarbonate and additional carbonic acid.]
And it can be paraphrased?
It is remarkable, very valuable phrase
Excuse for that I interfere … I understand this question. Is ready to help.