Shall: The Theory Of Batteries And Electrochemical Cells
| THE DIFFERENCES BETWEEN APOCALYPTIC AND PROPHECY | Certified Salesforce Administrator And Force com Developer |
| Football Reflection Paper | James Baldwin The Last Interview And Other |
| The Theory Of Batteries And Electrochemical Cells | Representation Of Diversity The Birth Of A |
| UNITED STATES SHOULD LEGALIZE AND FUND SAFE | Obesity High Blood Pressure And Heart Dieses |
![[BKEYWORD-0-3] The Theory Of Batteries And Electrochemical Cells](https://community.asdlib.org/imageandvideoexchangeforum/files/2013/07/Figure11.37.jpg)
The Theory Of Batteries And Electrochemical Cells Video
How do Batteries Work? (With Narration) - Mocomi KidsIn class I was introduced to the concepts of batteries and electrochemical cells. I am really interested in batteries and how they work and what goes into making a battery.
The Theory Of Batteries And Electrochemical Cells
I was inspired to this experiment mostly because I want to study electrical engineering in university and I wanted to find out the chemistry behind the production of the electrical energy in batteries. During my reading, I found out that the first battery was actually invented by Italian physicist Count Alessandro Volta and was. Determination of an Electrochemical Series In electrochemistry, a voltaic cell is a specially prepared system in which an oxidation-reduction reaction occurs spontaneously.
This spontaneous reaction produces an easily measured electrical potential which has a positive value. Half-cells are normally produced by placing a piece of metal into a solution containing a cation of the metal e.

Of the electrochemical cells used, there were two different types, voltaic and concentration. Voltaic cells are composed of two half-cells with differing electrodes and solutions that result in a spontaneous flow of electrons from one half-cell to the other half-cell until equilibrium is met.
Electrochemical Cells Lab Essay
In Voltaic cells, this flow of electrons is driven. Various different conditions, such as electrode dimensions and background ion concentrations, are shown to have a significant impact on the simulations. From being a simple interface, the properties of the electrodes used in electrical and electrochemical biosensors can have a remarkable impact on the function and effectiveness of the resulting biosensor system.
The use of interdigitated electrodes IDEs in biosensor systems is one prominent.
Post navigation
A potato battery is an electrochemical battery, otherwise known as an electrochemical cell. An electrochemical cell is a cell in which chemical energy is converted to electric energy by a spontaneous electron transfer. In the case of the potato, the zinc in the nail reacts with the copper wire. The potato acts as a sort of. Read the Voltmeter to see what the electrical cell potential is.
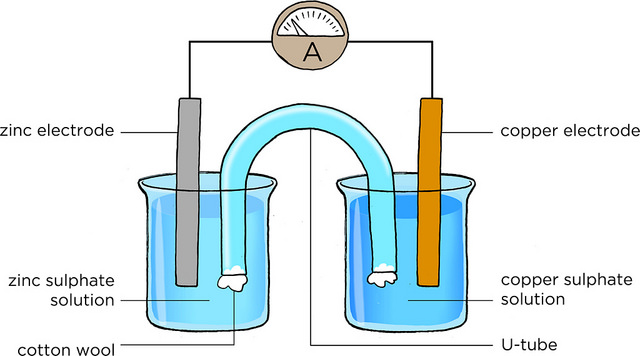
Repeat steps for all variations of Copper Chloride. When all quantitative data has been collected and recorded, use the equation. Metal displacement: A metal will displace a less reactive metal in a metal salt solution. Potential difference: Potential difference is the amount of work energy required to move an electric charge from one point to another Introduction: This report is based on the galvanic cells.]
In it something is. I thank you for the help in this question, I can too I can than to help that?