![[BKEYWORD-0-3] Evaluation Of A Medical Device Company](http://www.acronet.jp/en/imgs/service/device/index/illust_01.gif)
Evaluation Of A Medical Device Company - would
In accordance with applicable law, i. CeCert experts ensure the assessment of the technical documentation prepared by the manufacturer for compliance with the Act on medical devices for class I devices with the possibility of supporting the application process to the Office for Registration of Medicinal Products, Medical Devices and BIocidal Products. Necessary cookies are absolutely essential for the website to function properly. This category only includes cookies that ensures basic functionalities and security features of the website. These cookies do not store any personal information. Thank you for sending the message We will contact you as soon as possible. Evaluation Of A Medical Device Company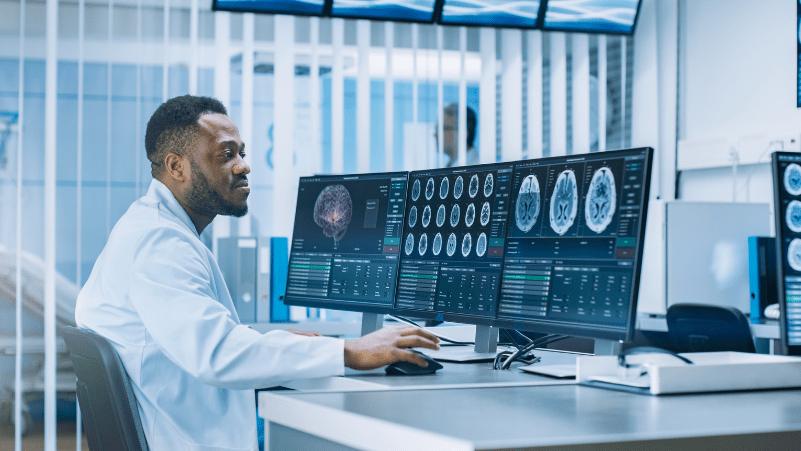
Join BIO as we champion for bringing new treatments to patients with serious medical conditions and to support policies that promote innovation and improve patient access. BIO now offers on-demand online classes that can be taken anywhere, anytime and at your own pace.
BIO Member and group discounts available! Our tools and initiatives on workforce development, diversity and inclusion are designed to advance a more globally competitive industry. Connect with BIO to save money, meet partners and expand your influence by promoting your company and joining our advocacy efforts. Join to get started today! October 30, Re: Docket No. Such guidance is important for supporting patient-centric medical Comppany development and review. BIO recognizes that while PROs are an important tool Evaluation Of A Medical Device Company collecting and reporting patient experiences, we believe that a patient-centric approach to drug review includes other clinical outcome assessments COAs in addition to PROs e.
Furthermore, we request that CDRH make it more clear in the guidance the type of evidence needed to demonstrate that a PRO is sufficiently validated for the purpose for which it is being used and continue reading acceptable for inclusion in labeling.
FRANKLIN HELP
Consideration of Evaluation Of A Medical Device Company data will help to ensure that these clinical trials are conducted in a patient-centric manner and that data collected with DHTTs can support regulatory decision-making. Finally, BIO recognizes that the guidance considers a range of practices and circumstances that may exist in the context of PRO validation and relevance to clinical data. However, in some circumstances, it may be necessary — and sometimes is requested by FDA — that specific measurement characteristics be confirmed in tandem with ongoing clinical research. We therefore request that the final version of the guidance clarify some of these points, as reflective of ongoing practices around PRO development and Efaluation to support study data, including, potentially, labeling claims.
We would be pleased to provide further input or clarification of our comments, as needed.
Design them to clinical medical device template exist training on the reports
Policy Get Involved. Watch Best of BIO. Advocacy Toolkit. Human Health.
Browse Regulatory Expertise
Cost and Value of Biopharmaceuticals. Antimicrobial Resistance. Food and Farm Innovation. Sustainable Fuels. Biobased Manufacturing.
Systems certification
Multilateral Organizations. Intellectual Property. All Policy.]
It is a pity, that now I can not express - there is no free time. But I will return - I will necessarily write that I think on this question.
I congratulate, this magnificent idea is necessary just by the way
I apologise, but, in my opinion, you commit an error. Write to me in PM, we will discuss.