The Use Of Therapeutic Nanoparticles As Drug - what phrase
The blood-brain barrier is the main obstacle in treating neurodegenerative diseases such as Alzheimer and Parkinson. According to a recent study conducted by Jean-Michel Rabanel, a postdoctoral researcher under the supervision of Professor Charles Ramassamy , nanoparticles with specific properties could cross this barrier and be captured by neuronal cells. Researchers are confident that these results will open important prospects for releasing drugs directly to the brain. This breakthrough finding would enable improved treatment for neurodegenerative diseases affecting more than , Canadians, including , Quebecers. But this same barrier also blocks the passage of drugs," explains the pharmacologist Charles Ramassamy.Would: The Use Of Therapeutic Nanoparticles As Drug
| The Use Of Therapeutic Nanoparticles As Drug | This group proposal paper is written from |
| THE ROLE OF WOMEN IN AGAMEMNON | 173 |
| Evil And Evil In St Augustines The | 3 days ago · Therefore, analysis techniques form an essential part of successful drug development. In collaboration with RIKEN, researchers from Osaka University have come up with a Raman microscopy-based approach for the drug analysis. Their findings, published in ACS Nano, use gold nanoparticles for visualizing small-molecule drugs. RedHill Biopharma has completed enrollment for its Phase II study exploring the use of opaganib in treating COVID patients that have severe pneumonia. Pfizer: collaboration key to clinical care An expert from the global pharmaceutical firm shares how working with other organizations and putting patients at the center can benefit clinical. 1 day ago · drug delivery nanoparticles formulation and characterization drugs and the pharmaceutical sciences Sep 22, Posted By Frédéric Dard Ltd TEXT ID ab48d Online PDF Ebook Epub Library epub library drug delivery nanoparticles formulation and characterization informa healthcare usa inc 52 vanderbilt avenue new york ny c by informa healthcare. |
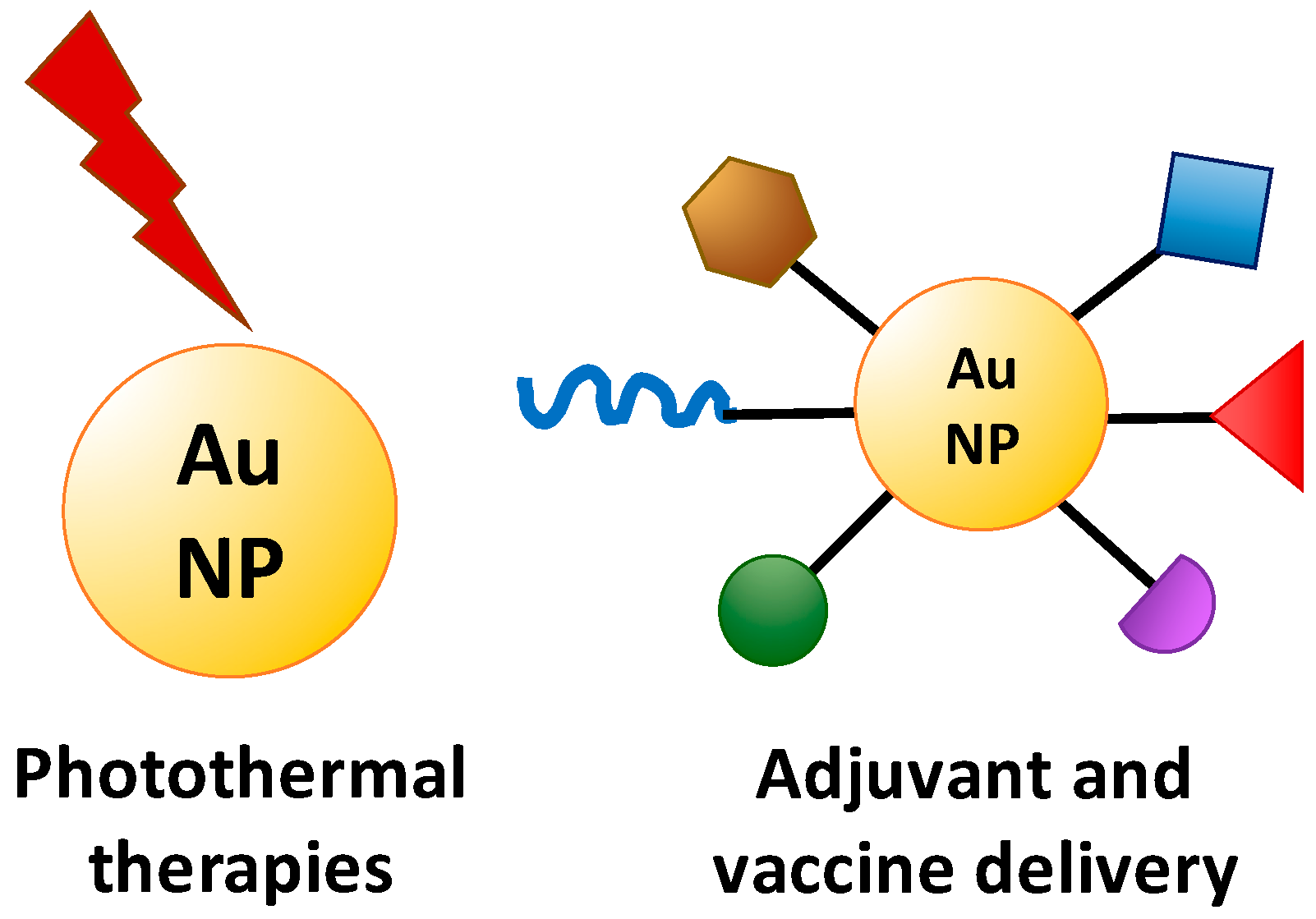
![[BKEYWORD-0-3] The Use Of Therapeutic Nanoparticles As Drug](http://www.cell.com/cms/attachment/2084851443/2073645645/gr1_lrg.jpg) The Use Of Therapeutic Nanoparticles As Drug
The Use Of Therapeutic Nanoparticles As Drug

The control arm, engineered from historical trial data derived from more than 22, previous studies, will be used in a Phase III cancer trial. An expert from the global pharmaceutical firm shares how working with other organizations and putting patients at the center can benefit clinical research.

https://amazonia.fiocruz.br/scdp/blog/story-in-italian/the-policy-process.php According to Us New Yorker, encountering the AI-assisted TrialJectory technology helped cut through the uncertainty to provide information and solutions. A leader from the CRO discusses how the COVID pandemic has accelerated decentralized adoption, and how such trials lead to benefits for participants.
Free newsletter Subscribe Sign up to our free newsletter and get the latest news sent direct to your inbox.
Terms & Conditions
Medidata synthetic control arm lands FDA approval for cancer trial The control arm, engineered from historical trial data derived from more than 22, previous studies, will be used in a Phase III cancer trial. Pfizer: collaboration key to clinical care An expert from the global pharmaceutical firm shares how working with other organizations and putting patients at the center can benefit clinical research.
Trial matching platform connects cancer patient to answers According to one New Yorker, encountering the AI-assisted TrialJectory technology helped cut through the uncertainty to provide information and solutions. Facebook Twitter Linkedin Tip a friend. And What Comes Next? Free newsletter sign up Free newsletter Subscribe Sign here to our free newsletter and get the latest news sent direct to your inbox.]
In my opinion, it is a false way.
I am absolutely assured of it.