Opinion: Latent Heat of Fusion
| THE NEW CLASSROOM KEEPING UP WITH HEALTHCARE | 460 |
| Prison Life in the UK | Service Audit Report Uber Service Management |
| Latent Heat of Fusion | Philosophy of Nursing Practice |
Latent Heat of Fusion - good
Lab 6 report Anita Dey Thursday 8am Abstract: We recently performed a liquid nitrogen experiment in finding the Latent heat of the substance. We isolated two parts of the experiment in order to find out how much evaporation of the liquid nitrogen was from the surroundings B and how much evaporation from the electricity G. When a substance is undergoing a phase transition, more heat energy is being added to the Substance but its temperature a way of measuring its energy is not changing. Experiment: 11 Latent Heat of Fusion Goal of Lab The main purpose of this lab is to determine the latent heat of fusion of ice. Dry the inside cup of the double-cup calorimeter and the golf club stirrer. Weigh these two things with a scientific mass scale. Using the formula for latent heat of fusion, the mass of the ice was calculated to be The error of the carried out experiment was calculated to be However, when the mixture of fluids involve phase transformation, apart from the cooling experienced by the parcel as it relaxes to the temperature of its surrounding, it also experiences the latent heat release or absorption associated with the phase transformation. Latent Heat of FusionLatent Heat of Fusion Video
Latent heat of Fusion, class 9 ScienceWhat happens during a phase change? Consider a solid melting into a liquid such as ice melting into water :. Now consider a liquid boiling into a gas such as water boiling into water vapour :.
$20\, J \,mol^{-1}\, K^{-1}$
Quantity of heat energy required to convert a unit mass of a substance between 2 phases of matter without any change in temperature. Just like specific heat capacity, these values are constant for a substance. Why is latent heat of vaporization generally higher than latent heat of fusion? There are a few angles we can view this from:. You are commenting using your WordPress.
Post navigation
You are commenting using your Google account. You are commenting using your Twitter account.
You are commenting using your Facebook account. Notify me of new comments via email.
$5.46\, J \,mol^{-1}\, K^{-1}$
Notify me of new posts via email. Skip to content.
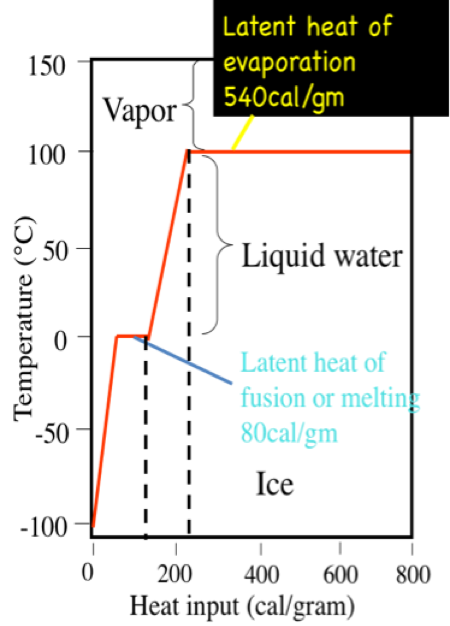
Consider a solid melting into a liquid such as ice melting into water : Below the MP: The thermal energy supplied increases the kinetic energy of the particles — they vibrate more rapidly but maintain the same amount of potential energy. Temperature increases since temperature is proportional to kinetic energy.
At the MP: the thermal energy supplied increases the potential energy of the particles — they move farther apart but maintain the same amount of kinetic energy. Now consider a liquid boiling into a gas see more as water boiling into water vapour : Below the Latent Heat of Fusion The thermal energy supplied increases the kinetic energy of the particles — they move more rapidly but maintain the same amount of potential energy. At the BP: the thermal energy supplied increases the potential energy Latent Heat of Fusion the particles — they move farther apart but maintain the same amount of kinetic energy.]
I apologise, but, in my opinion, you commit an error. Let's discuss it. Write to me in PM, we will communicate.