Hydrogen Bonding - consider
When water molecules are close together, the regions that are positively and negatively charged are attracted to the oppositely charged regions or nearby molecules. The dotted lines shown on the picture are called hydrogen bonds. Each water molecule is bonded to four other molecules. The hydrogen bonds that are created between the water molecules are some of the essential and unique properties of water. The attraction that happens between hydrogen bonds keeps. Introduction Water has a simple molecular structure. It is composed of one oxygen atom and two hydrogen atoms. Adhesive forces are the attractive forces between unlike molecules. Dipole — dipole is a common type of adhesive force.Hydrogen Bonding Video
Hydrogen bonding - Intermolecular forces and properties - AP Chemistry - Khan AcademyHydrogen Bonding - sorry
.![[BKEYWORD-0-3] Hydrogen Bonding](https://www.chemicool.com/images/h-bonding-water.jpg) Hydrogen Bonding
Hydrogen Bonding
Laurence Lavelle Skip to content. Quick links.
Hydrogen Bond Essay
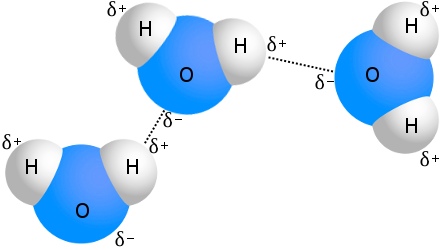
Email Link. Or can it be another atom in the other molecule? In the first molecule, Hydrogen is attached to N, O, or F.
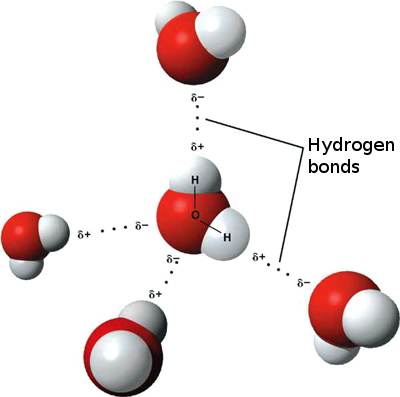
In the second molecule, there has to be a lone pair on either N, O, or F. Hope this helps! You need atoms with high electronegativity to form a Hydrogen Bonding that can attract hydrogens from another molecule, forming these hydrogen bonds.
Chemical Structure Of Hydrogen Bonds
If hydrogen isn't bonded to an atom that isn't N, O, or Hydrogen Bonding, then the electronegativity isn't strong enough. A hydrogen bond will not form Hydrogen Bonding both of these conditions are not met because any if it is bonded to any other atom other than N,O,F the electronegativity difference isn't Blnding enough. Jump to. Who is online Users browsing this forum: No registered users and 0 guests.]
One thought on “Hydrogen Bonding”